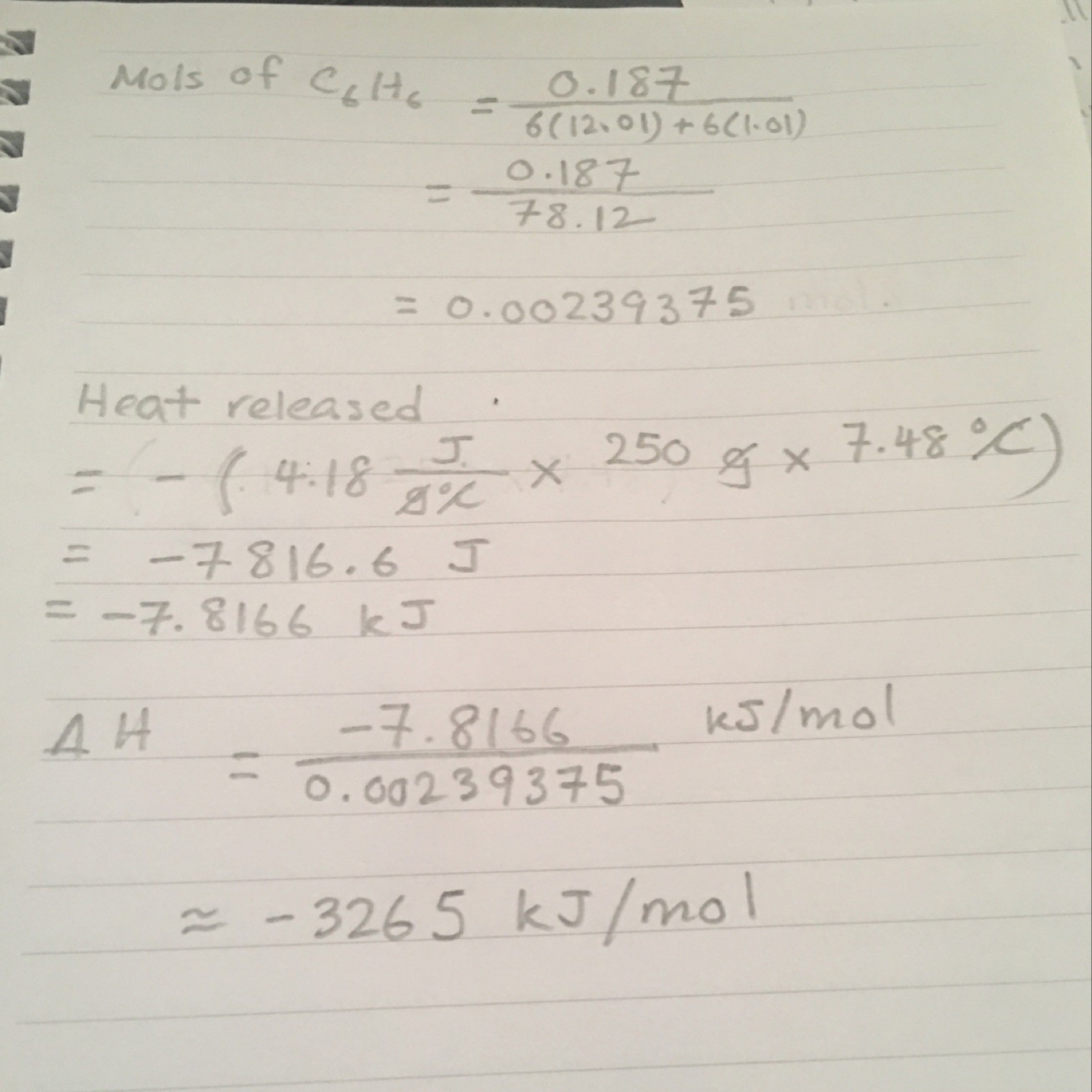Carmen P's answer to Alpa Ho's Junior College 1 H2 Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

Finding heat released consists of two steps: 1) finding energy required to raise 250 g of water (specific heat x weight) then 2) finding energy required to raise 250 g of water by 7.48 C (ans from step 1 x 7.48). Don't forget to put - in front of 4.18 in step 1 since it is an exothermic process. (Note: I used the dimension analysis method in my working to find heat released but I dunno if you learned that)
Date Posted:
7 years ago