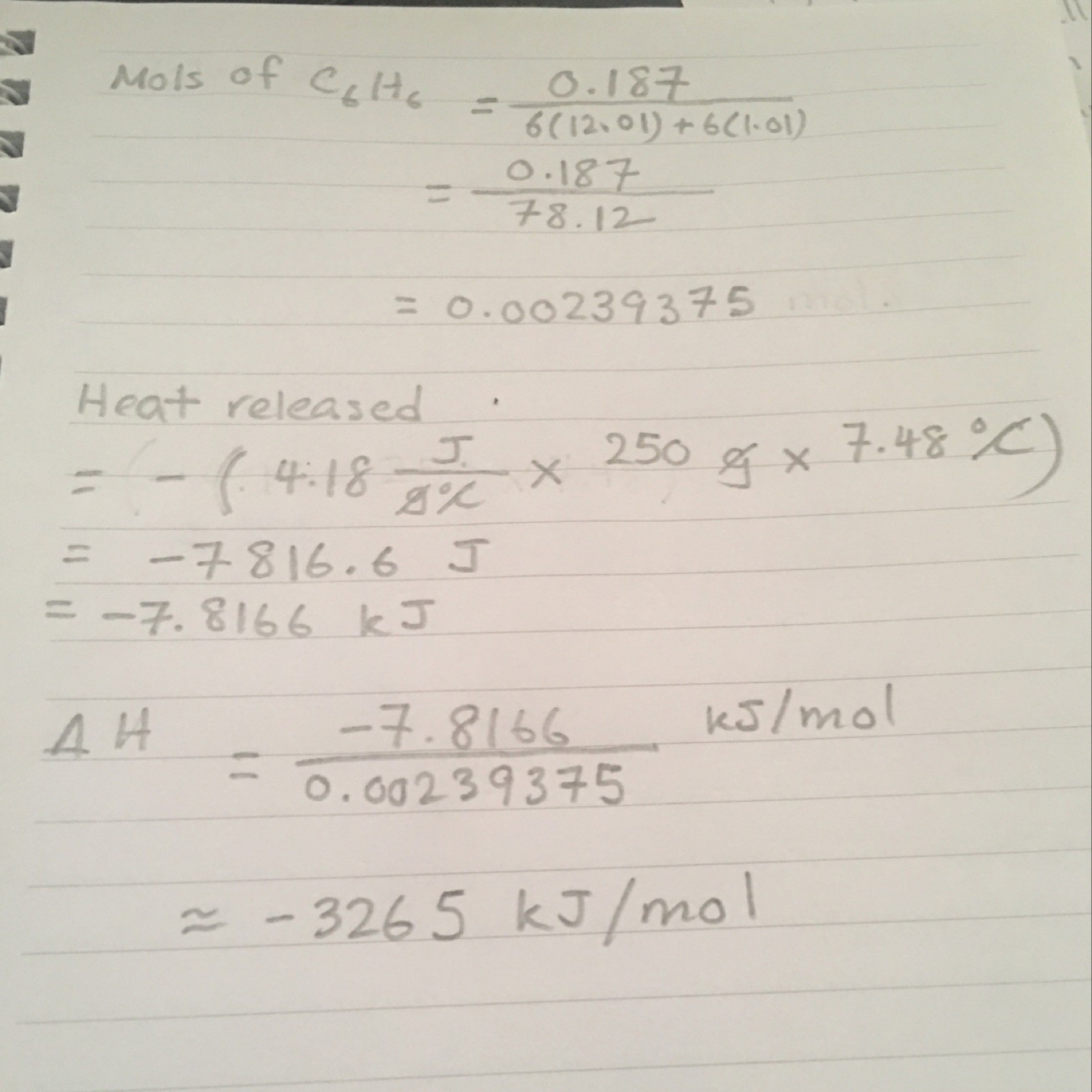Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 1 | H2 Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.
H2 Chemistry.
Topic on Thermochemistry - calculate the combustion enthalpy for benzene in kilojoule per mole.
Answer is: ΔH = -3265 kJ/mol (not sure what do i do first)
See 1 Answer