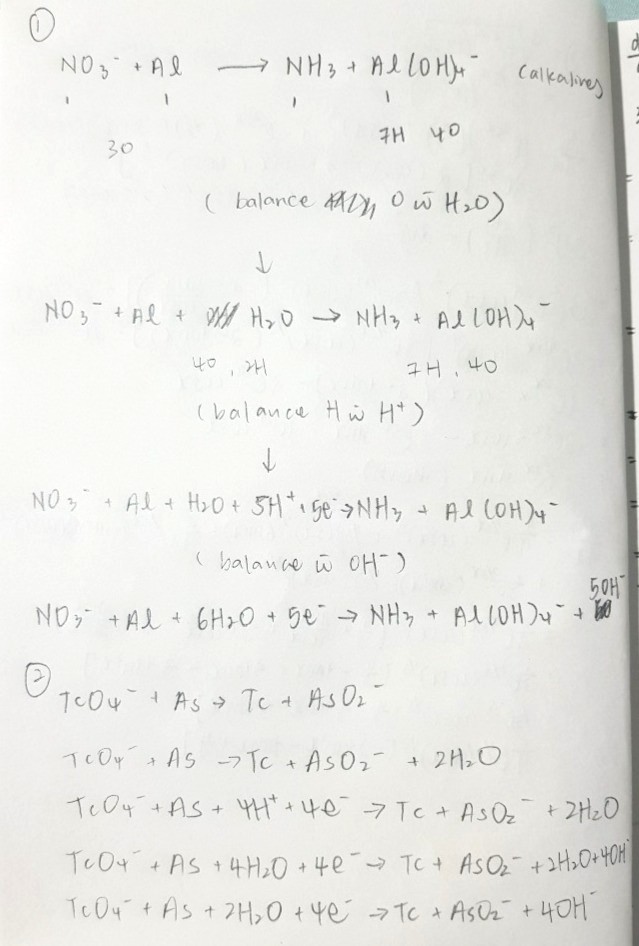Bonnie's answer to Sonia's Junior College 2 H2 Maths Singapore question.
done
1 Upvotes
clear 0 Downvotes

Not 100% sure if this is correct but i used the same steps for both chem equations by
1) balancing O with H2O
2) balancing H with H+
3) balancing charges with electrons
4) removing H+ with OH-
1) balancing O with H2O
2) balancing H with H+
3) balancing charges with electrons
4) removing H+ with OH-
Date Posted:
5 years ago
Yup, , looks correct to me