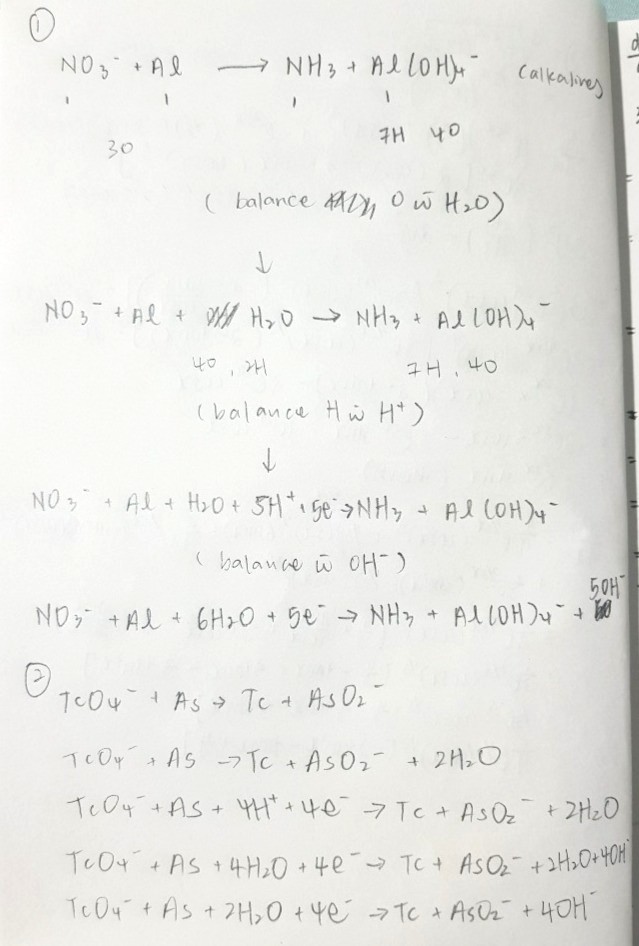Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 2 | H2 Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

#H2Chem
Can someone help me with balancing these 2 redox equations? And could u include detailed working/explanation because my answer is wrong but i don’t know where i gone wrong .. Thaks so much:)
N and Al are already balanced.
So balance O first using H2O.
① NO3- + Al + H2O → NH3 + Al(OH)4-
Notice that the left hand side has 5 less H than the right hand side.
If you add H2O to left side and OH- to right side, you're adding one more H+ overall on the left as compared to the right.
This helps to lower the difference in H on both sides.
To reduce the difference from 5 to 0, add 5 more H2O to the left and 5 more OH- to the right
② NO3- + Al + 6H2O→ NH3 + Al(OH)4- + 5OH-
Now balance the electron count.
③ NO3- + Al + 6H2O + 5e→ NH3 + Al(OH)4- + 5OH-
See 1 Answer

1) balancing O with H2O
2) balancing H with H+
3) balancing charges with electrons
4) removing H+ with OH-