Arnold K H Tan's answer to Annela's Secondary 4 Chemistry Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

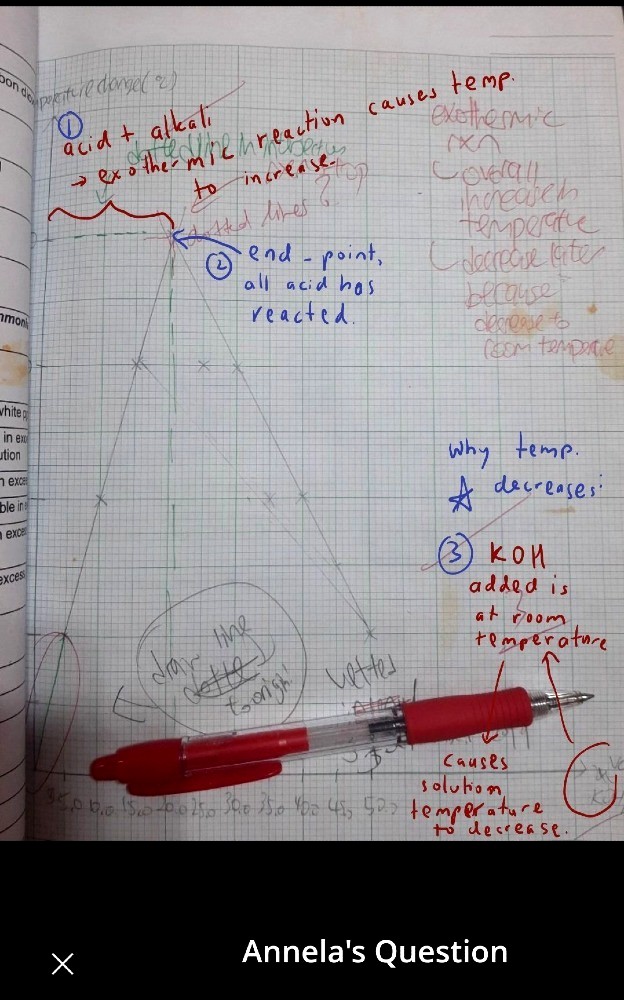
Unfortunately, both J's and Eric's comments are incorrect for the short time frame of a titration experiment.
Date Posted:
4 years ago
The excess KOH added after the end point for the reaction, cools the solution. KOH is at room temperature. Similarly for endothermic reaction, the lowest temperature (below ambient room temperature) indicates when the limiting reactant has been used up. As more of the excess reactant is added, the temperature rises to room temperature, as the excess reactant added is at room temperature.
Interesting. I took it that the horizontal axis was time and KOH was added to neutralisation/equivalence point. If excess KOH is added then yes, the KOH’s room temperature is a more significant factor than the air’s room temperature.
Arnold’s solutions are correct since the horizontal axis represents the volume of KOH added and not time.
Annela, I suggest an “extrapolation” of the straight lines by dotted lines instead of solid lines.
Arnold’s solutions are correct since the horizontal axis represents the volume of KOH added and not time.
Annela, I suggest an “extrapolation” of the straight lines by dotted lines instead of solid lines.