Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.

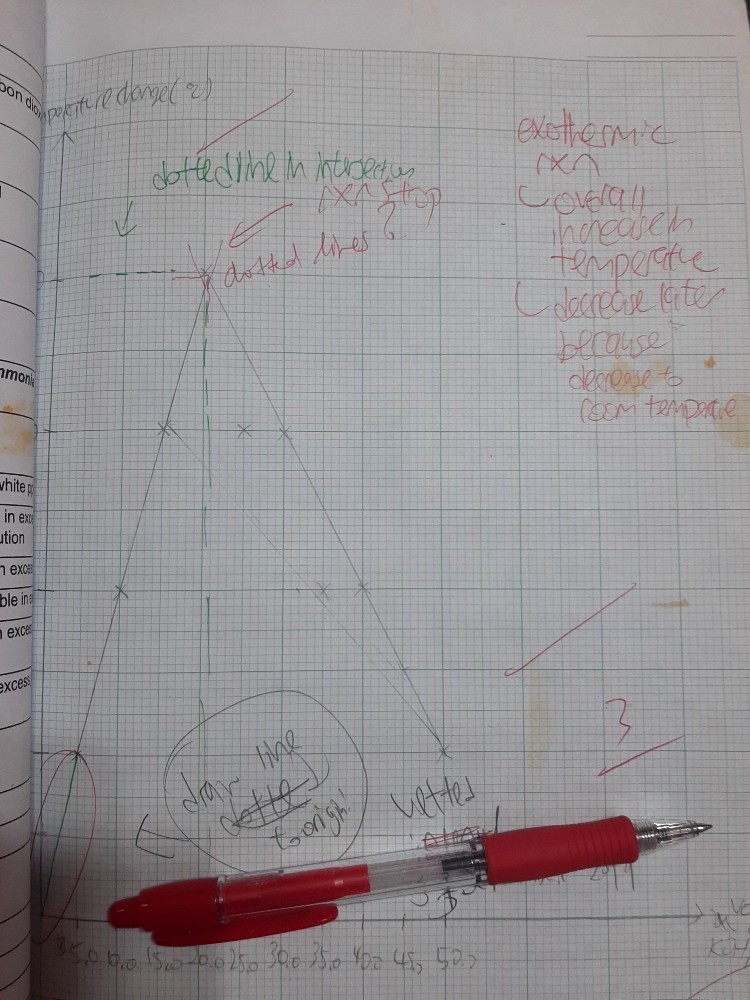
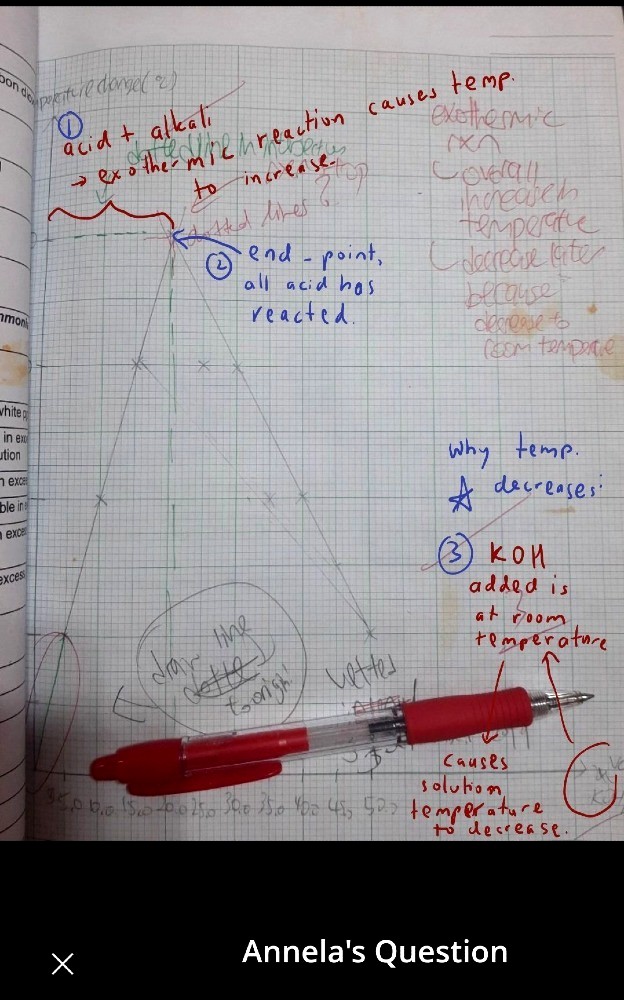
This is exothermic graph.I understand the intersection point means the reaction end but why the temperature decreases? Is it decreases to room temperature?
Aso for endothermic graph, the temperature decreases but yet increases again?
Can someone explain to me why so?
The reaction starts out at room temperature and rises in temperature (being exothermic). Once the reaction concludes, there is no more release of heat energy from the reaction. Eventually, hot air spreads to cooler areas by diffusion to allow the temperature between the reaction and the surroundings to reach an equilibrium temperature.
It’s a similar idea for the endothermic version.
Think of why the water in a kettle cools down after boiling.
See 1 Answer

Arnold’s solutions are correct since the horizontal axis represents the volume of KOH added and not time.
Annela, I suggest an “extrapolation” of the straight lines by dotted lines instead of solid lines.