Eric Nicholas K's answer to Ria's Secondary 3 E Maths Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
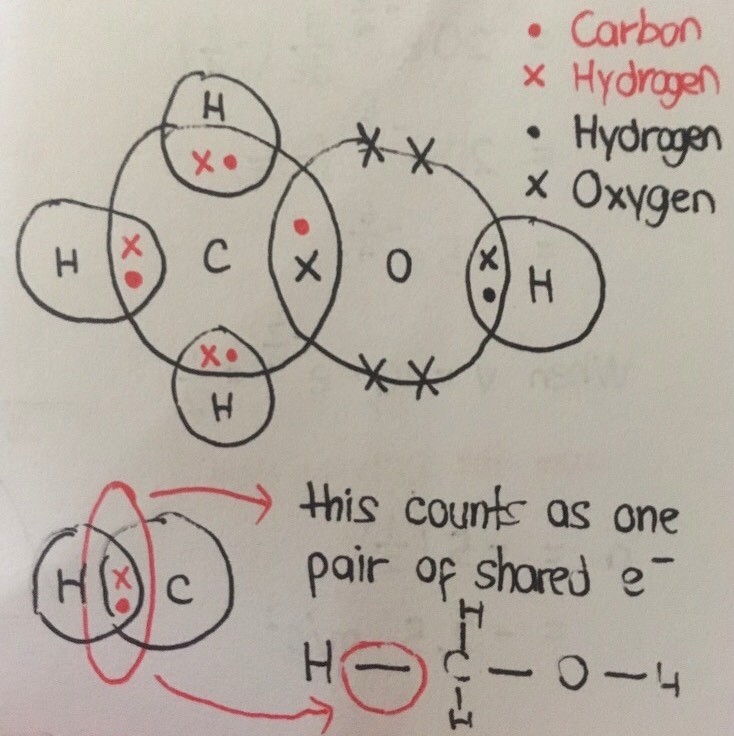
The dot and cross diagram that I have drawn is a “fully expanded” form of the given chemical structure.
Each line represents two bonding electrons. So altogether there are 5 x 2 = 10 electrons used in bonding, leaving behind 4 unbonded electrons from the oxygen atom.
You will learn in Sec 4 that CH3OH is the chemical structure of an alcohol.
Each line represents two bonding electrons. So altogether there are 5 x 2 = 10 electrons used in bonding, leaving behind 4 unbonded electrons from the oxygen atom.
You will learn in Sec 4 that CH3OH is the chemical structure of an alcohol.
Date Posted:
4 years ago
The electrons in the overlapped circles are the 'shared electrons'. Recall that electrons are being shared in covalent bonding between atoms of non-metals.