Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

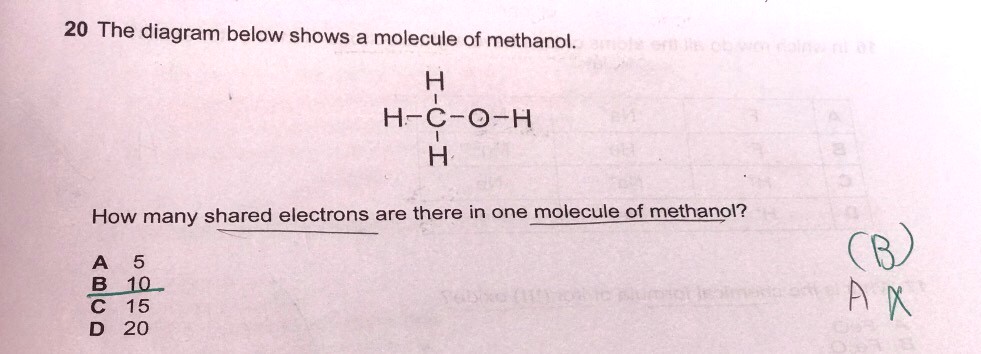
Can someone please explain to me why for this question the answer is 10 I really do not understand what is shared electrons and what do they mean by how many shared electrons are there in one molecule what is one molecule? please someone explain to me this qn thank you very much appreciated
Do you see 5 lines/dashes in the diagram?
Those are the covalent bonds betwen the atoms. In each covalent bond, there are two electrons shared. So each line/dash represents a covalent bond of 2 shared electrons.
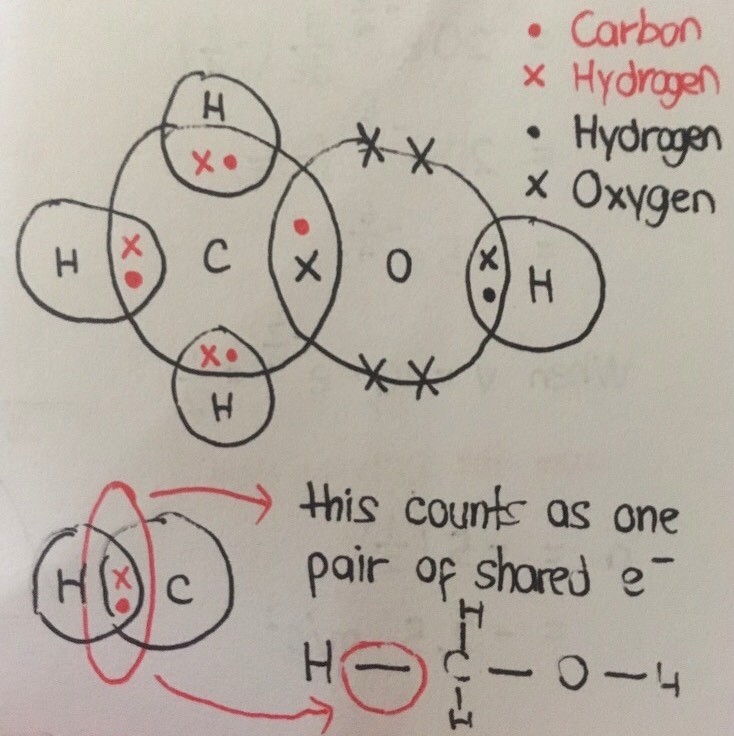
The atoms share electrons in order to attain the stable noble gas /octet configuration of 8 valence electrons (except hydrogen, where it is 2 electrons/duplet instead)
The oxygen has 6 valence electrons . It shares 1 electron with each of the 2 adjacent hydrogen atoms to form 1 covalent bond each. The hydrogen atoms themselves share their only valence electron with oxygen in forming that bond.
The 4 remaining valence electrons of oxygen + 2 covalent bonds x 2 shared electrons per bond = 8 electrons
Carbon has 4 valence electrons. It shares 1 electron with each of the 4 adjacent hydrogen atoms to form a total of 4 covalent bonds.
4 covalent bonds x 2 shared electrons each bond = 8 electrons.
Since there are 5 bonds in this diagram,
5 x 2 shared electrons for each bond = 10 shared electrons.
See 1 Answer
Each line represents two bonding electrons. So altogether there are 5 x 2 = 10 electrons used in bonding, leaving behind 4 unbonded electrons from the oxygen atom.
You will learn in Sec 4 that CH3OH is the chemical structure of an alcohol.