Arnold K H Tan's answer to kim Yeon Jae's Secondary 3 A Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

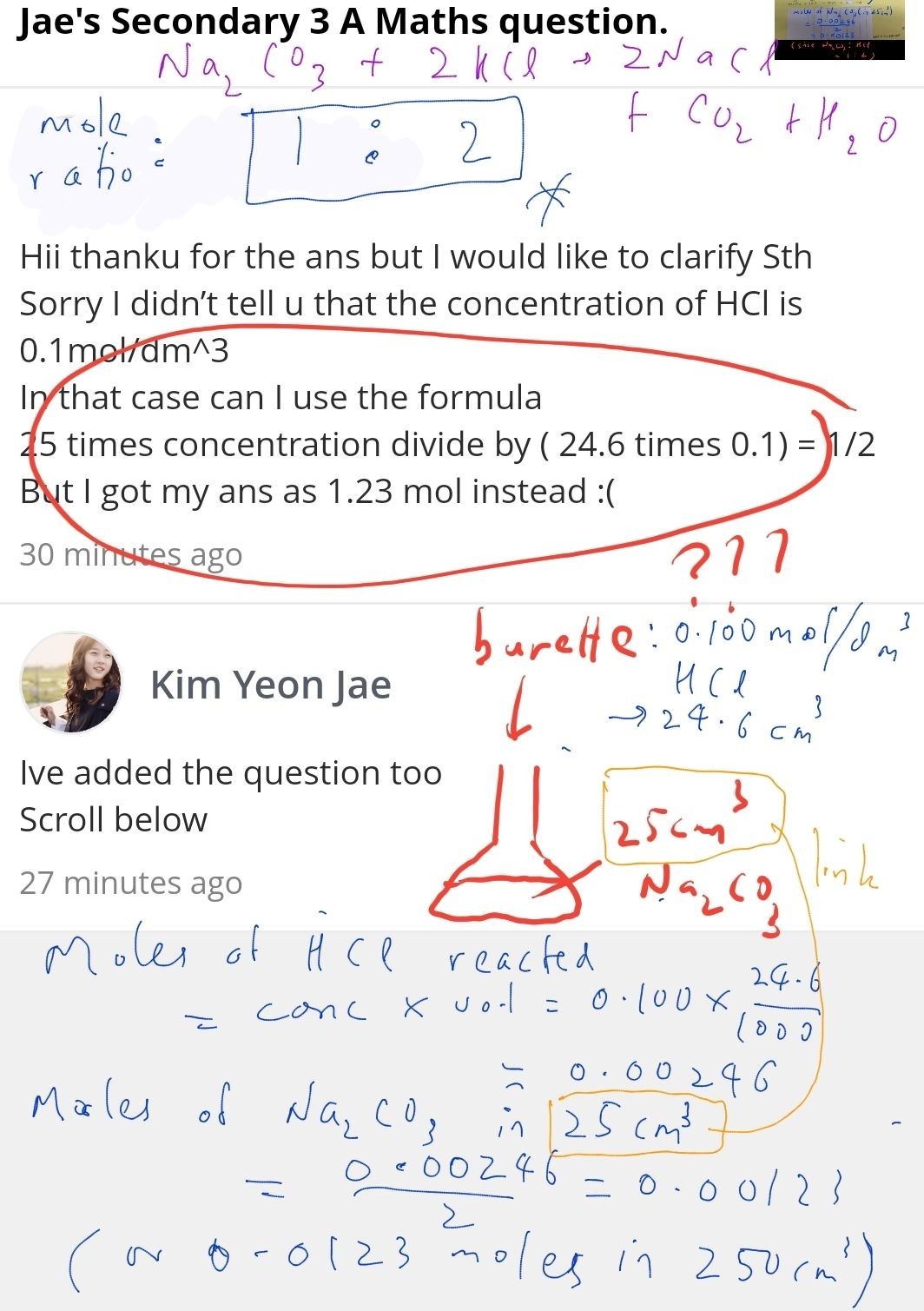
The question asked for the number of moles of Na2CO3 in the 25cm3 Na2CO3 solution that was titrated against the 0.100 mo/dm3 HCl. From the balanced equation, 1 mole of Na2CO3 needs 2 moles of HCl for complete reaction. Thus 0.00246 moles of HCl reacted with 0.00246 ÷ 2 = 0.00123 moles Na2CO3 in the 25cm3 Na2CO3.
Date Posted:
6 years ago