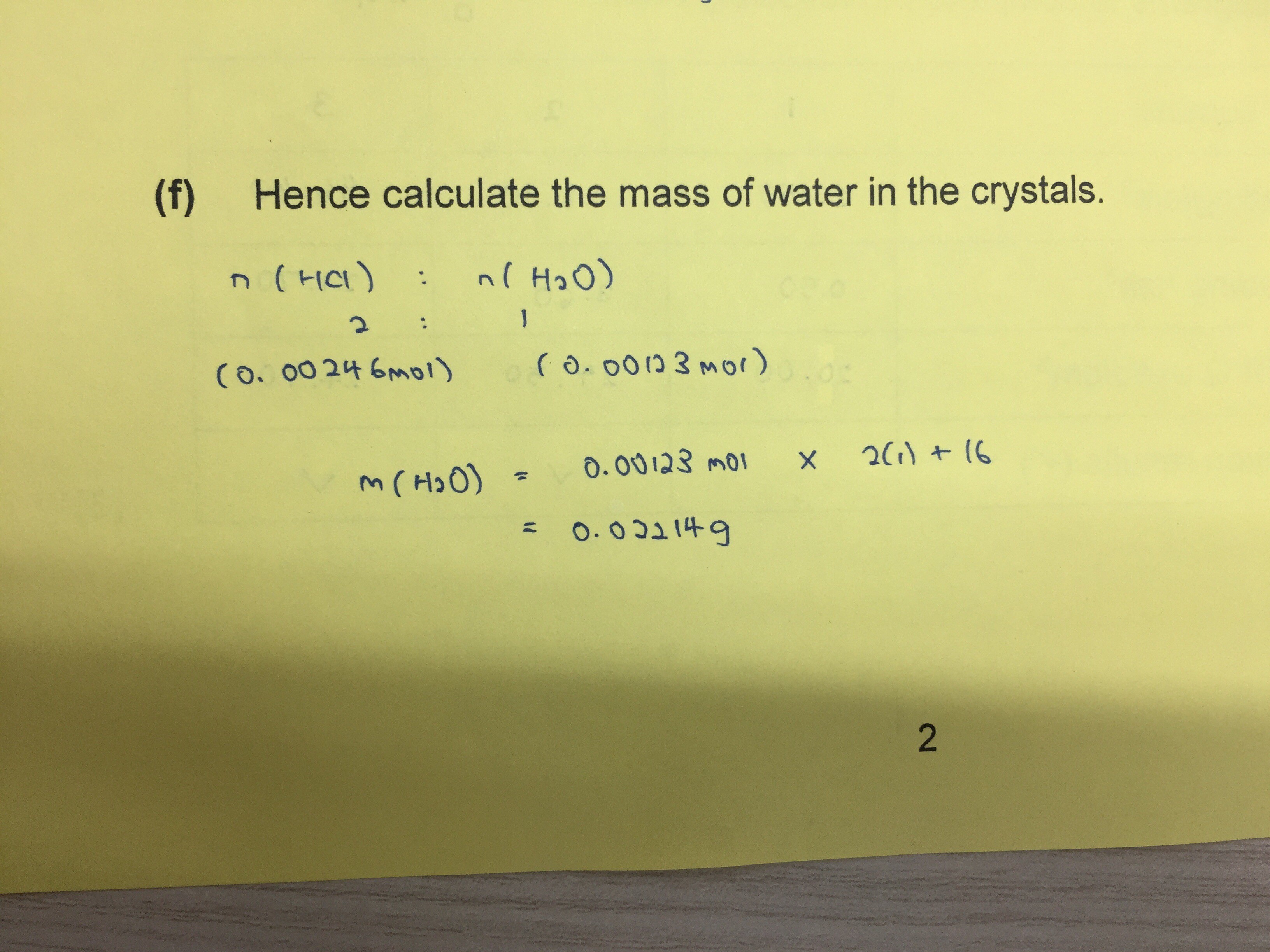Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 5 Answers
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

I tutor Chemistry, Physics and Biology, as well as A/Emath on the sidelines. Will attend to questions on these subjects, time permitting. Little or no time during the exam period, as I will be busy working to prepare my students to score well.
Date Posted:
6 years ago
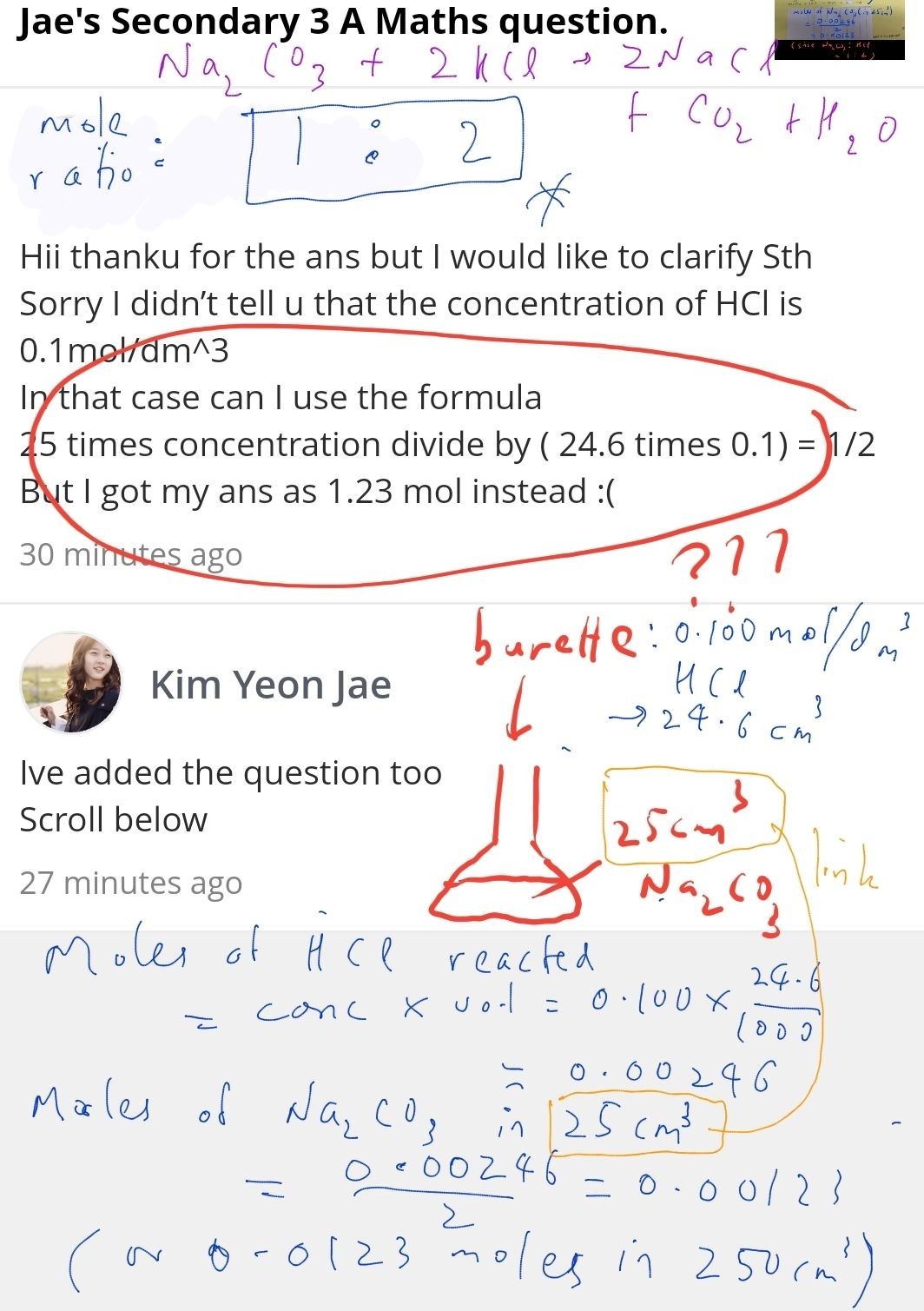
Hii thanku for the ans but I would like to clarify Sth
Sorry I didn’t tell u that the concentration of HCl is 0.1mol/dm^3
In that case can I use the formula
25 times concentration divide by ( 24.6 times 0.1) = 1/2
But I got my ans as 1.23 mol instead :(
Sorry I didn’t tell u that the concentration of HCl is 0.1mol/dm^3
In that case can I use the formula
25 times concentration divide by ( 24.6 times 0.1) = 1/2
But I got my ans as 1.23 mol instead :(
Ive added the question too
Scroll below
Scroll below
The 1.23 you calculated is the concentration of Na2CO3 in mol/dm3, which was not required by the question. The question wanted you to determine the number of moles in the 25cm3 Na2CO3 solution used for the titration.
Ooo I see I understand alr but I have 1 last qn
Below
Below
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

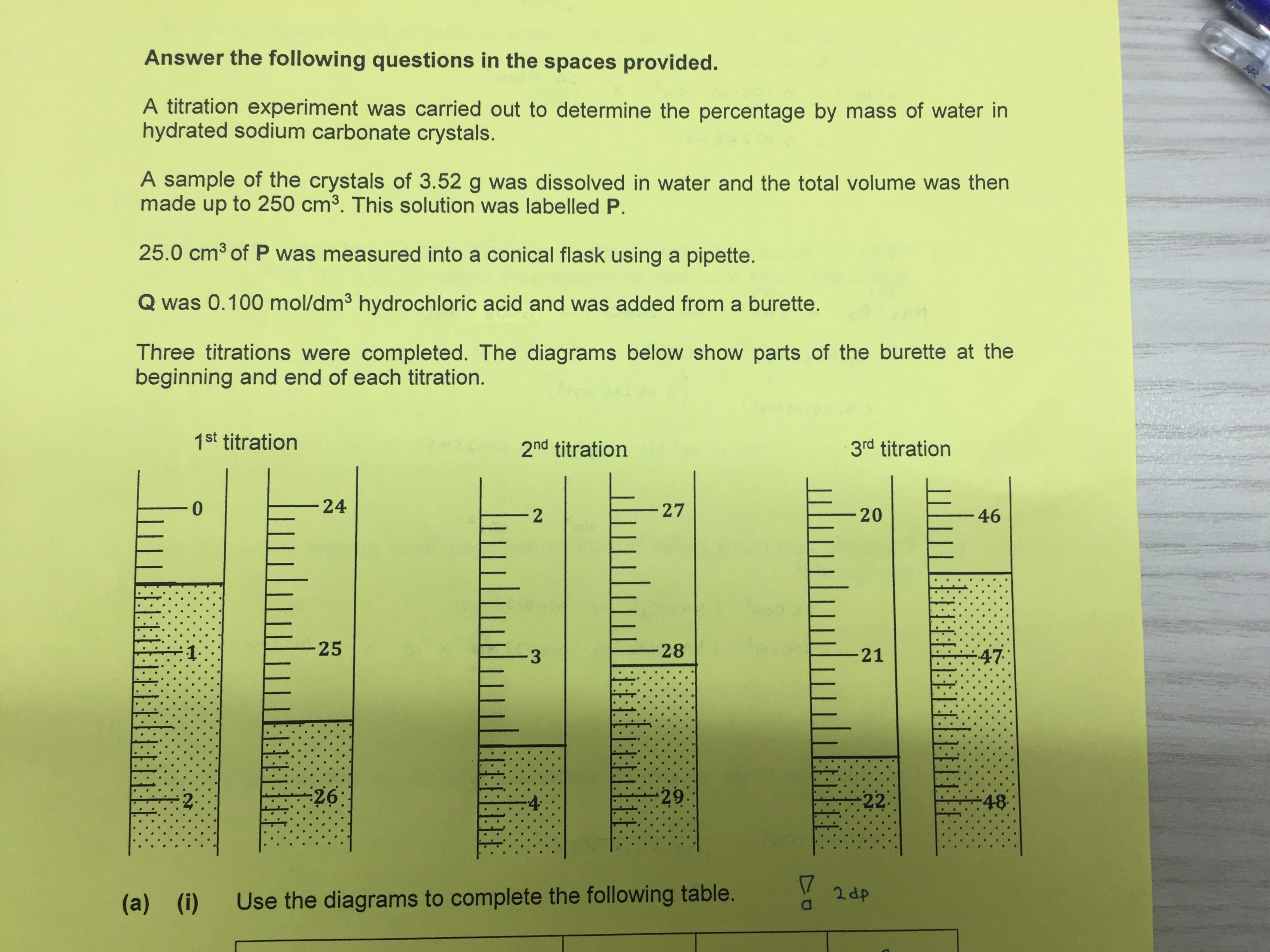
This is the qn
Date Posted:
6 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

The question asked for the number of moles of Na2CO3 in the 25cm3 Na2CO3 solution that was titrated against the 0.100 mo/dm3 HCl. From the balanced equation, 1 mole of Na2CO3 needs 2 moles of HCl for complete reaction. Thus 0.00246 moles of HCl reacted with 0.00246 ÷ 2 = 0.00123 moles Na2CO3 in the 25cm3 Na2CO3.
Date Posted:
6 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

I don’t think this is correct as I haven’t made use of the 3.52g of crystal that the qn has provided.
Date Posted:
6 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

The mass of water the question is referring to is the mass of water of crystallisation. It benefits me that I already know the formula for hydrated sodium carbonate.
Date Posted:
6 years ago