PhysChemTutor's answer to Jonathan's Secondary 4 Chemistry Singapore question.
Thank you very much!
https://www.bbc.co.uk/bitesize/guides/zqwmxnb/revision/3
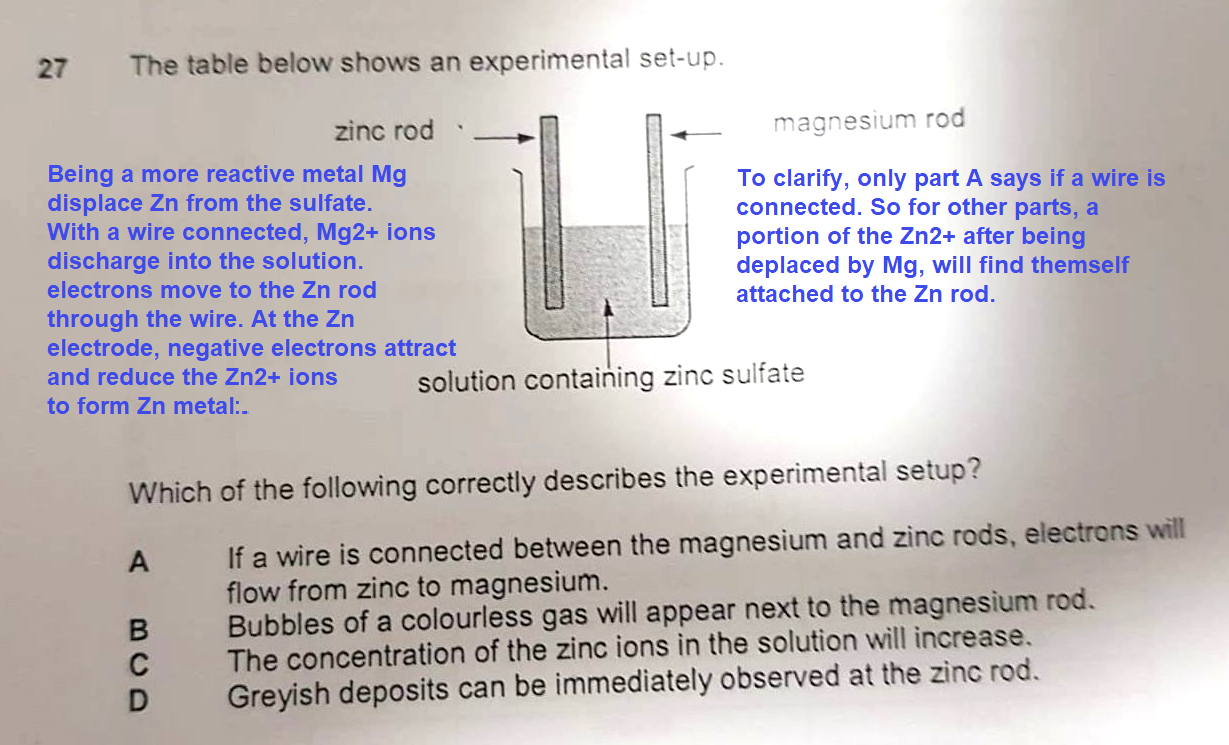
C would have been correct if the word was 'magnesium' instead of 'zinc', since magnesium metal (Mg) on the rod would displace zinc ions (Zn²+) in solution and become Mg²+
i.e magnesium acts as the reducing agent and itself gets oxidised
zinc acts as the oxidising agent and itself gets reduced.
C would have been correct if the word was 'magnesium' instead of 'zinc', since magnesium metal (Mg) on the rod would displace zinc ions (Zn²+) in solution and become Mg²+
i.e magnesium acts as the reducing agent and itself gets oxidised
zinc acts as the oxidising agent and itself gets reduced.