Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

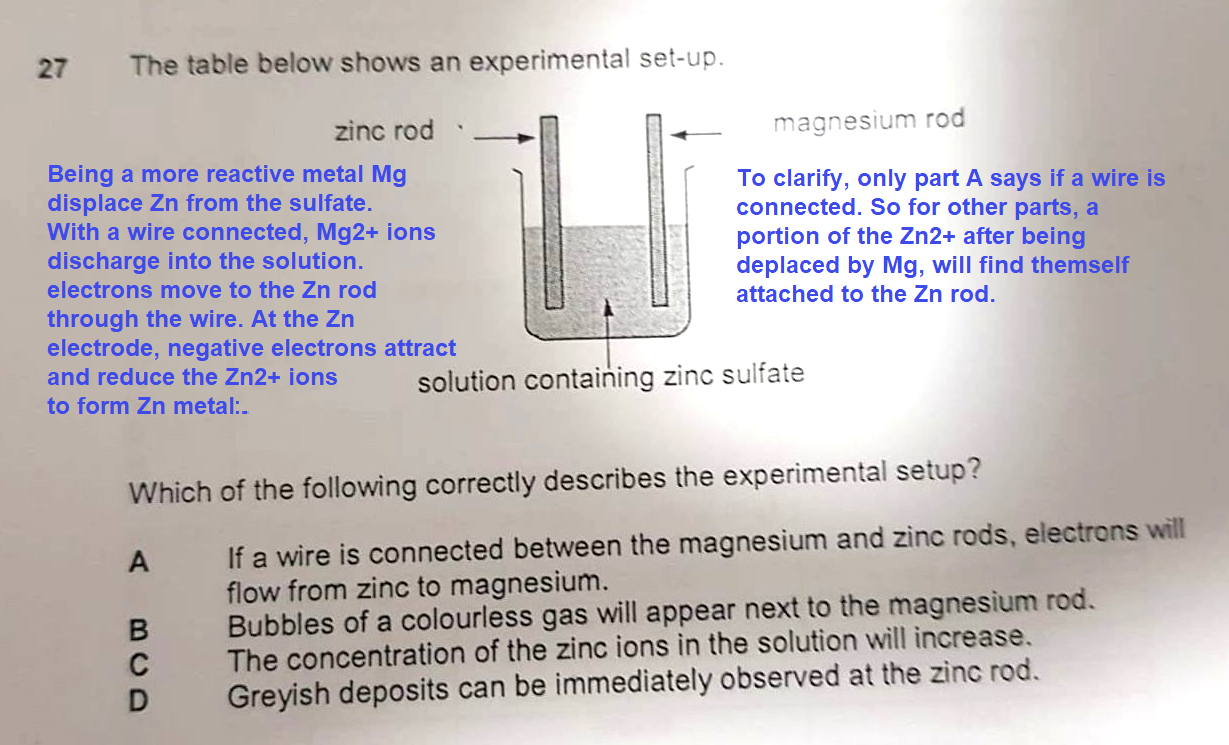
Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.

May I know since the set up does not have a wire how do electrons travel into the zinc electrode?
Sincere thanks once again!
See 1 Answer
C would have been correct if the word was 'magnesium' instead of 'zinc', since magnesium metal (Mg) on the rod would displace zinc ions (Zn²+) in solution and become Mg²+
i.e magnesium acts as the reducing agent and itself gets oxidised
zinc acts as the oxidising agent and itself gets reduced.