
 360 Tutoring Program
360 Tutoring Program
*If you qualify, the Free 360 Tutoring Program will be extended to you at no extra charge.
On top of the tutor you are getting, ManyTutors will provide unlimited free tutors to help you with any questions you may have for other subjects. Just snap a photo of your homework, post, and someone will provide the solution for free.
You will get a complimentary premium account on Ask ManyTutors that allows you to ask English, Chinese, Malay, Math, Science, Geography and History homework questions free of charge.
Under the 360 Program, the tutor will receive a different rate from what you are paying to ManyTutors.
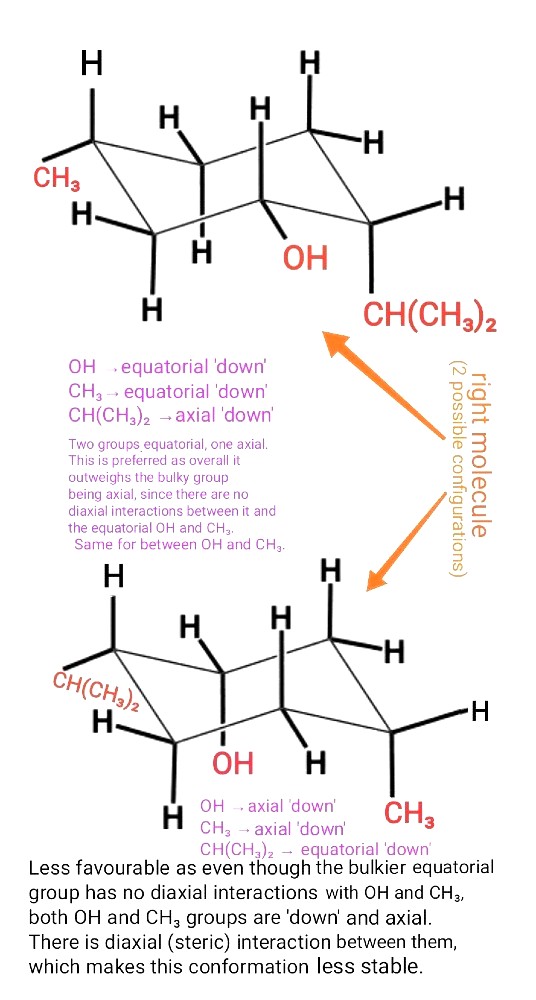

'There are no diaxial interactions between it and the equatorial OH and CH3 groups'
The 'it' here refers to the bulky group.
This doesn't mean that the bulky group has no diaxial interactions with the axial Hs.
For the configuration below, you have 1 OH and 1 CH3 group having 1,3-diaxial interactions with 1 axial H, as well as with each other.
OH and CH3 are both bigger substituents than a single H (hydrogen is the smallest atom in the entire Periodic table).
So the combined interactions (i.e steric) between the OH,CH3 and axial H will be bigger and outweigh the interactions between bulky group and two small Hs.