Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 2 | H3 Maths
2 Answers Below
Anyone can contribute an answer, even non-tutors.

Sorry there isnt any option for chemistry. But may i know what is wrong? What are the steps in thinking process for this question?
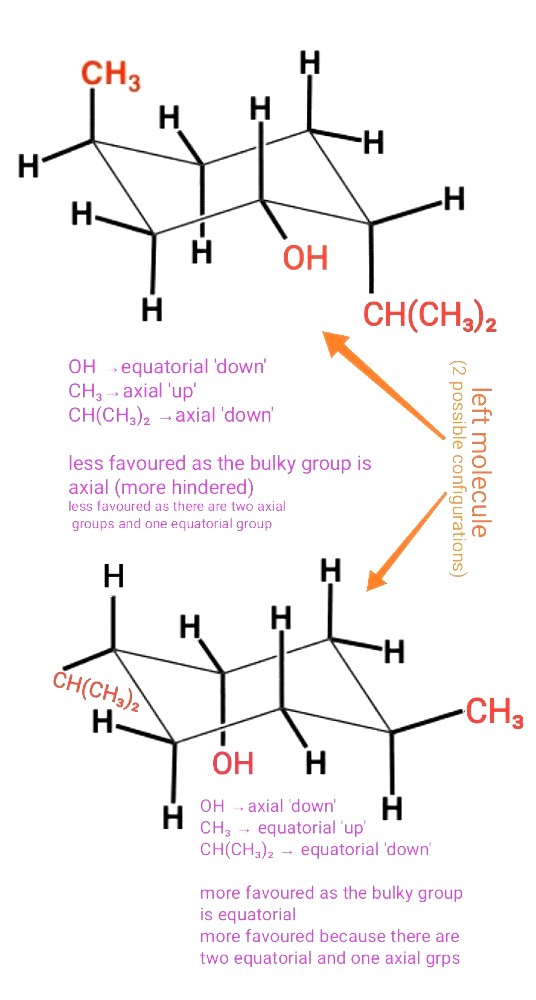
Those bonds with dashed lines or longer dashes (∥∥∥∥∥∥) are 'down'
Those with wedges are 'up'
From here, you then decide if it's equatorial or axial
From the looks of the second structure, it's probably the case that the bulkier group and the OH group should not be co-equatorial to minimise steric hindrance/interactions.
See 2 Answers
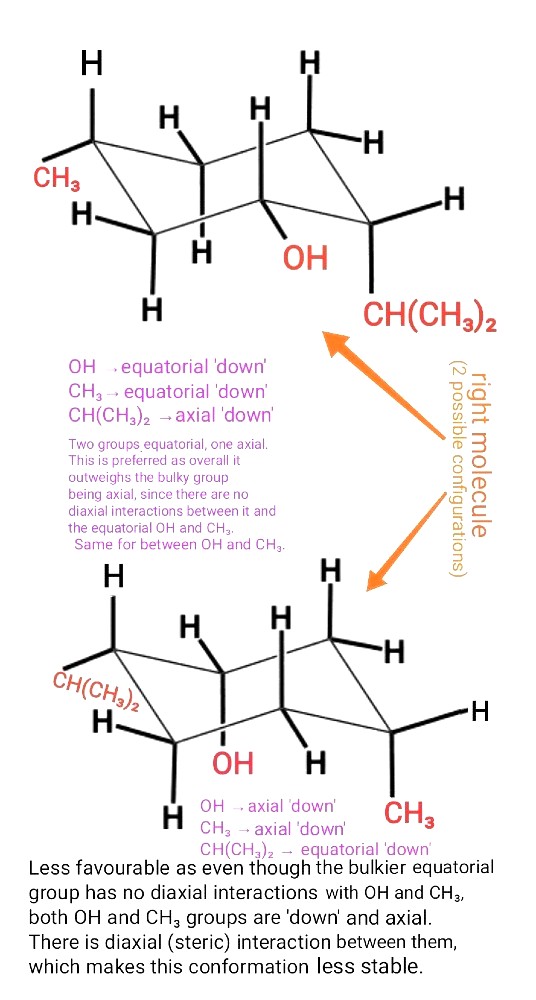
'There are no diaxial interactions between it and the equatorial OH and CH3 groups'
The 'it' here refers to the bulky group.
This doesn't mean that the bulky group has no diaxial interactions with the axial Hs.
For the configuration below, you have 1 OH and 1 CH3 group having 1,3-diaxial interactions with 1 axial H, as well as with each other.
OH and CH3 are both bigger substituents than a single H (hydrogen is the smallest atom in the entire Periodic table).
So the combined interactions (i.e steric) between the OH,CH3 and axial H will be bigger and outweigh the interactions between bulky group and two small Hs.