Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.

Can i ask why is the answer B?
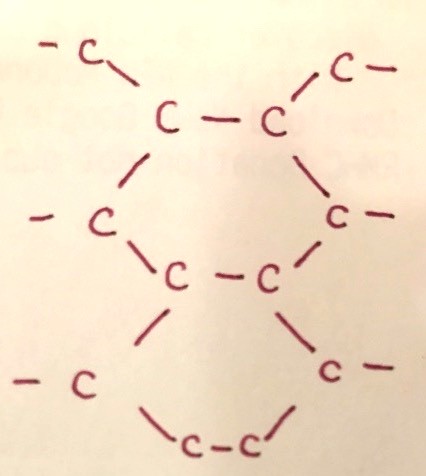
You can do an online read up on graphite to see its structure.
See 1 Answer
Observe that the carbon is only attached to three other neighbouring carbon atoms (three lines drawn out from each carbon). This means that each carbon atom only uses three of its four valence electrons for bonding, leaving behind one electron which is free to move around (since they have nowhere else to bond to).
This explains why graphite can conduct electricity.
I had to do a Google search on this to see the structure.
https://www.chemguide.co.uk/atoms/structures/giantcov.html
You can see in the pictorial that the carbon atoms (in black) are each bonded to four oxygen atoms while the oxygen atoms are each bonded to two carbon atoms.
This article also lists out the cases for graphite and diamond.
Poly(ethene) can be thought of as a "never-ending alkane", where four-way bonding occurs in almost every carbon present.