Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
5 Answers Below
Anyone can contribute an answer, even non-tutors.

Can you help me with qn 34 and 36? Thank u
Q34 is basically a list of who is the “overall champion” of the competition.
Q36 I look later.
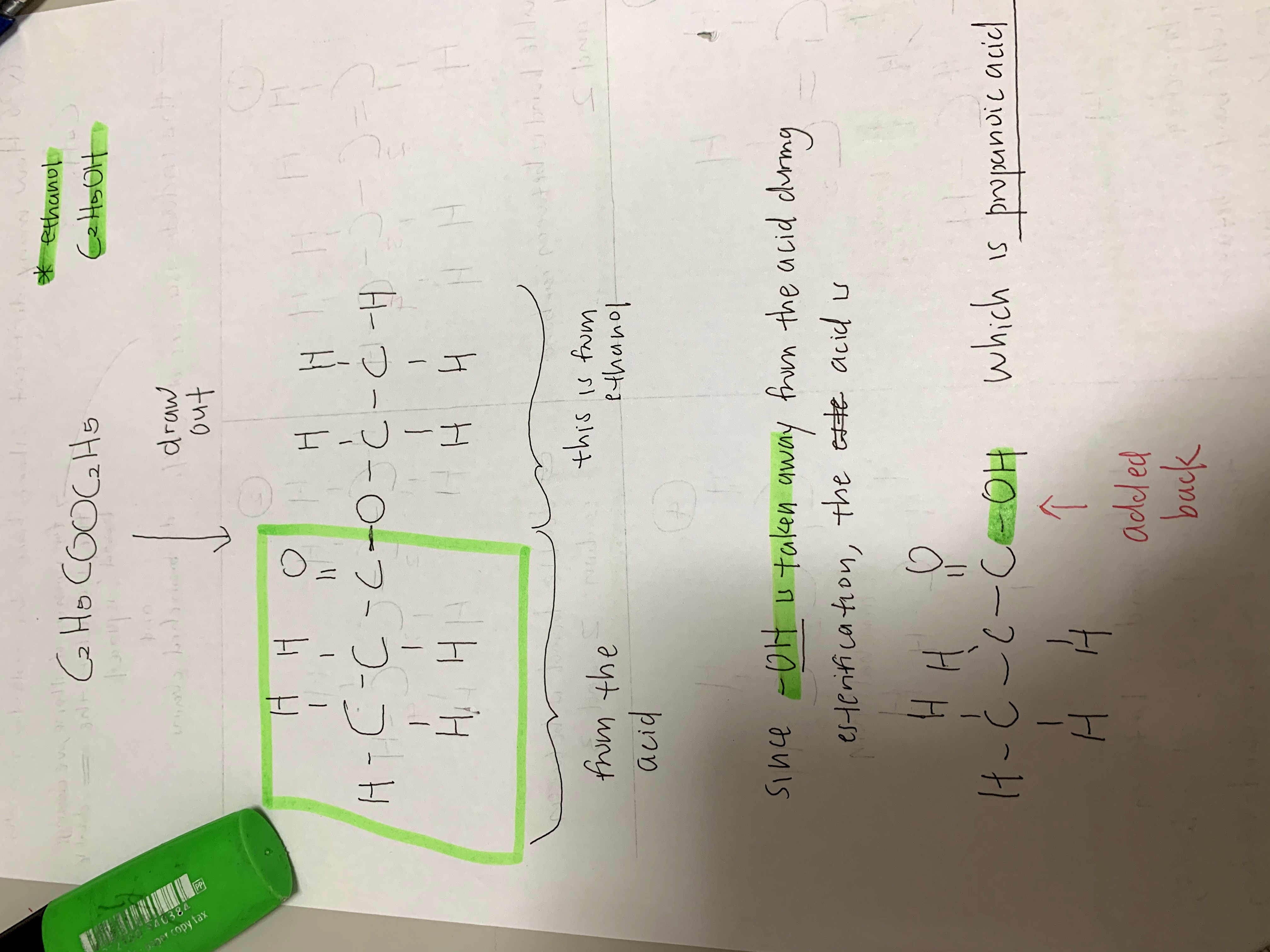
The straight chain carbon arrangement for a related alkane goes
C - C - C - C
Now we attempt to put the relevant double bonds.
Putting the double bond between the first two carbons is no different from putting the double bond between the third and fourth carbons, because they are symmetrically the same.
Putting the double bond between the middle two carbons gives a different compound altogether. Isomers, to be more precise.
All the other elements are purely hydrogens, so there is no other possible combination.
So there are two possible structures.
Y and Z do not win W2+, so W is stronger than Y and Z.
Y fails to win anyone, so Y is the least reactive.
Z wins only Y, so Z is stronger than Y but weaker than X and W.
So, from most reactive to least reactive, it will be X (overall champion), followed by W (second place), Z (third place), Y (fourth place).
Aso, your drawn structure for Q36 is incorrect since 5 lines are drawn out of the same carbon, which is not possible as carbon only has 4 valence electrons.
See 5 Answers
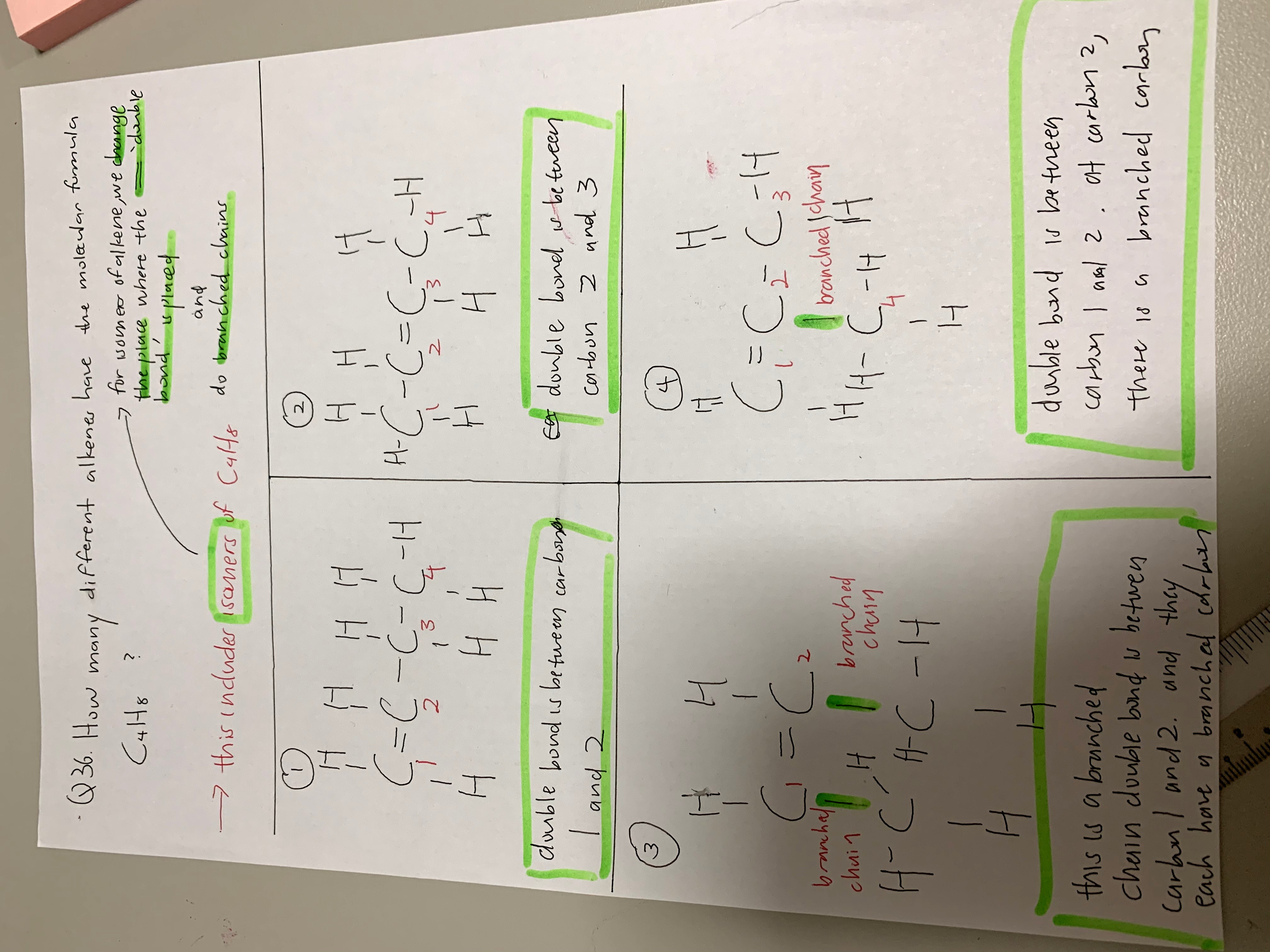
Missed one configuration with C = C - C where the fourth carbon is attached to the centre C above.
Configuration 1 is C = C - C - C.
Configuration 2 is C - C = C - C. This can be further broken down into the different cis- and trans- configurations, but the O Levels do not touch this at all. This is only covered in the A Levels.
Configuration 3 is C = C - C where the fourth carbon is branched out to the second carbon.
In the A Levels this is taken to be four configurations. In the O Levels, this is taken to be only three configurations.
——————
@ Han Song
There are only four possible isomers for this. The fifth one is a repetition of one of the isomers.
The relevant link to refer is http://web.pdx.edu/~wamserc/C334F01/H5ans.htm where the different isomers are listed out.
Option A is the straight chain version with the double bond between the first two carbons.
Options B and C are the straight chain versions with the double bond between the middle two carbons. Option B is for the cis- configuration (on the same side) while option C is for the trans- configuration (on the opposite wing). Option D is the branched version which I initially missed.
@ Annela
Do take note that cis- and trans- isomerisation is not covered in your syllabus. You can take it that there are three isomers (when learnt in the O Levels).
The syllabus in the A Levels distinguishes between the two configurations, so in effect there are actually four possible isomers.

EDIT: Just crossed check with online sources and you are right.

Because the carbon at the “bottom” sides can be placed on the left side and right side without making much difference.
As an O Level student I would put 3 isomers. As an A level student I would put 4 isomers.