Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
3 Answers Below
Anyone can contribute an answer, even non-tutors.

Can someone please explain to me how to do qn 7 and 8?
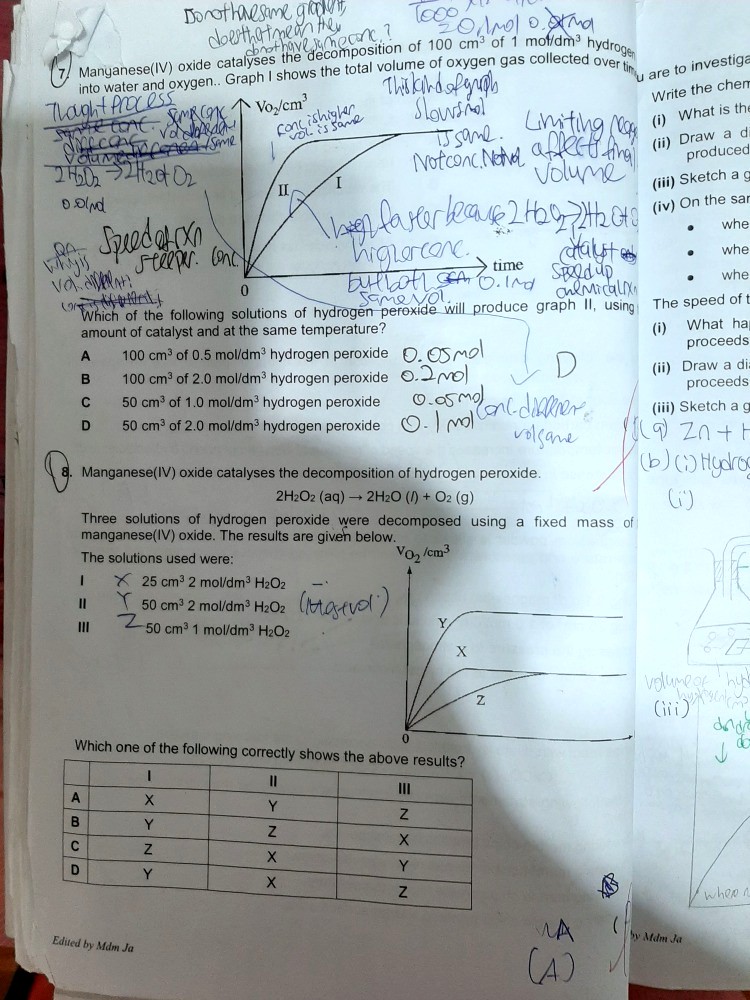
Q7 should be D, since the amount of gases formed will depend on the amount of peroxide decomposed.
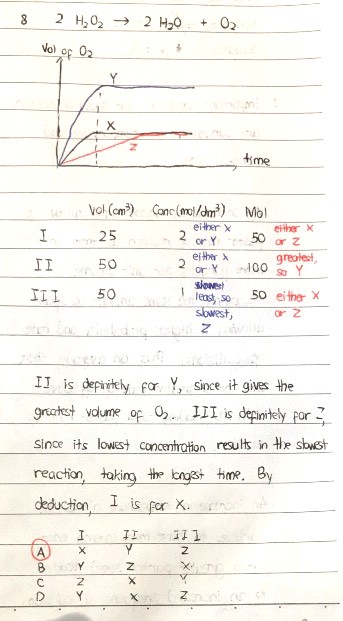
For Q8, I and III form the two graphs X and Z. Upon closer inspection, I is for X because the higher concentration allows a greater number of molecules at any one point of time to collide with others and the frequency of collisions increases. This is true even though the number of miles of H2O2 in both cases is the same.
Will write these up for you later.
See 3 Answers
A consequence of this is that solution II contains 50/1000 x 2 = 0.1 mol of H2O2 while solution III contains 50/1000 x 1 = 0.05 mol of H2O2. Clearly solution II has twice as much H2O2 as solution III. As a result, solution II will liberate twice as much oxygen gas (from the decomposition reaction) as solution III.
So in this case, we cannot say that having the same volume of H2O2 results in graphs of the same height, simply because the amount of contents dissolved in the same volume of liquid is not the same in those cases; in fact, the graph is only directly related to the number of moles of O2 formed. The more the number of moles of H2O2 decomposed, the more the number of moles of O2 generated and hence the more the volume of O2 generated. This translates to a higher final position in the graph.
If we observe the axes carefully, the vertical axis represents the volume of O2 generated, which is directly proportional to the number of moles of O2 generated (and of course the number of moles of H2O2 decomposed). As a result, solution II should result in a graph that reaches a maximum height which is twice that of solution III.
Hence, graph Y corresponds to solution II.
Between X and Z, the slower one, which is Z, must have come from the solution with the lowest concentration, which is solution III.
That leaves graph X for solution I.
The graph ends at the same height but graph II ends earlier than graph I, indicating that the number of moles of O2 gas produced is the same, thereby telling us that the number of moles of H2O2 decomposed must be the same in both cases but the concentration used in II is higher than in I.
D is the best option for Q7.
Wait for Q8.