Lynn's answer to abc's Secondary 3 Chemistry question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

Hope this answers :)
Date Posted:
7 years ago
If you don't understand what the question wants:
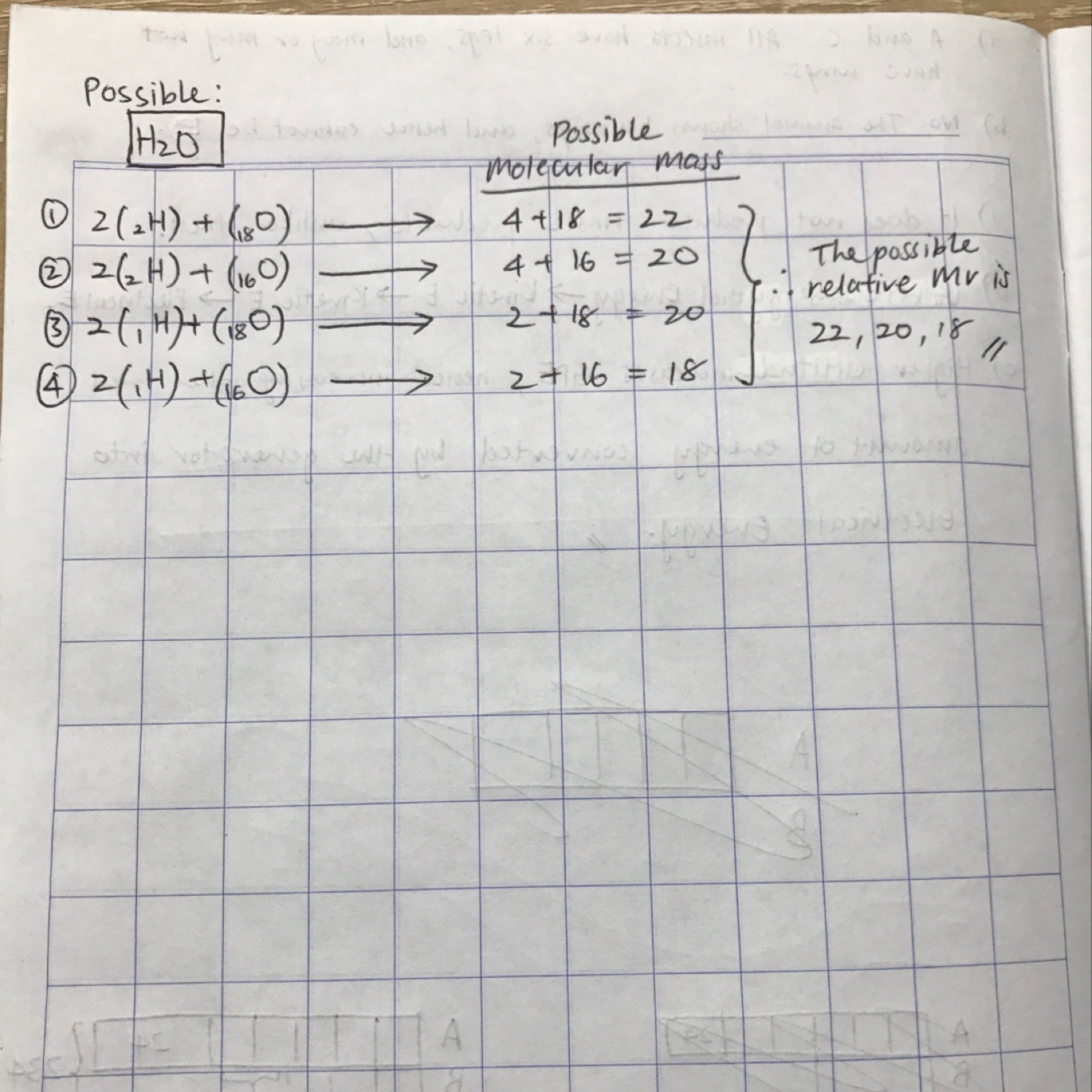
It's asking for the possible combinations of H₂O being formed by the Hydrogen and Oxygen isotopes.
Note that despite the number of neutrons changing, the chemical properties (i.e. Bonding to form H₂O) remains same.
Hence the mass of each isotope is different, as each neutron has a mass of 1g/mol of element.
So, you find the possible combinations of H and O isotopes.
Then you calculate possible Mr of each combination.
That is your answer. :)
Jiayou! ^^
It's asking for the possible combinations of H₂O being formed by the Hydrogen and Oxygen isotopes.
Note that despite the number of neutrons changing, the chemical properties (i.e. Bonding to form H₂O) remains same.
Hence the mass of each isotope is different, as each neutron has a mass of 1g/mol of element.
So, you find the possible combinations of H and O isotopes.
Then you calculate possible Mr of each combination.
That is your answer. :)
Jiayou! ^^