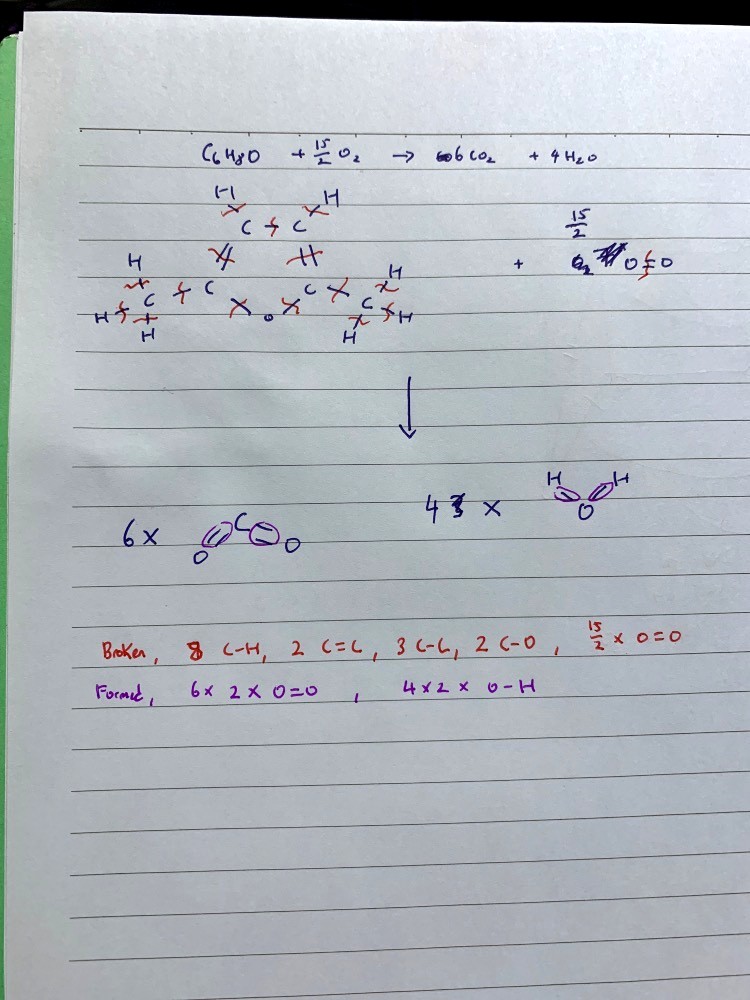
Brian Chiong's answer to Sonia's Junior College 1 H2 Maths Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Bonds broken are in red, bonds formed are in purple. I used 15/2 O2 for this equation but to balance it out remember to x2 everything to get 2 C6H8O + 15 O2 —> 12 CO2 + 8 H2O
Hope this helps!
Hope this helps!
Date Posted:
4 years ago
Thank u so much for ur fast reply! May i know if u have any tips on how to draw the structure of the molecule ? I get confused especially when there are many constituent elements
For organic molecules, to draw the structure you could try starting from one carbon first, then drawing all the bonds from that one carbon before moving onto the next atom, rather than looking at the whole molecule which may be confusing. After you are done drawing everything out, count the atoms you have to make sure it fits with the chemical formula. Usually the one that is missed out when converting from skeletal to structural would be the H atoms. But at the end of the day you just need to keep practicing drawing the structure if its something that troubles you, over time it will get easier and easier to convert between your skeletal and structural formulas :) Just go to google and search for some random molecules and try drawing it out.
Thank you so much! Truly appreciate it :)