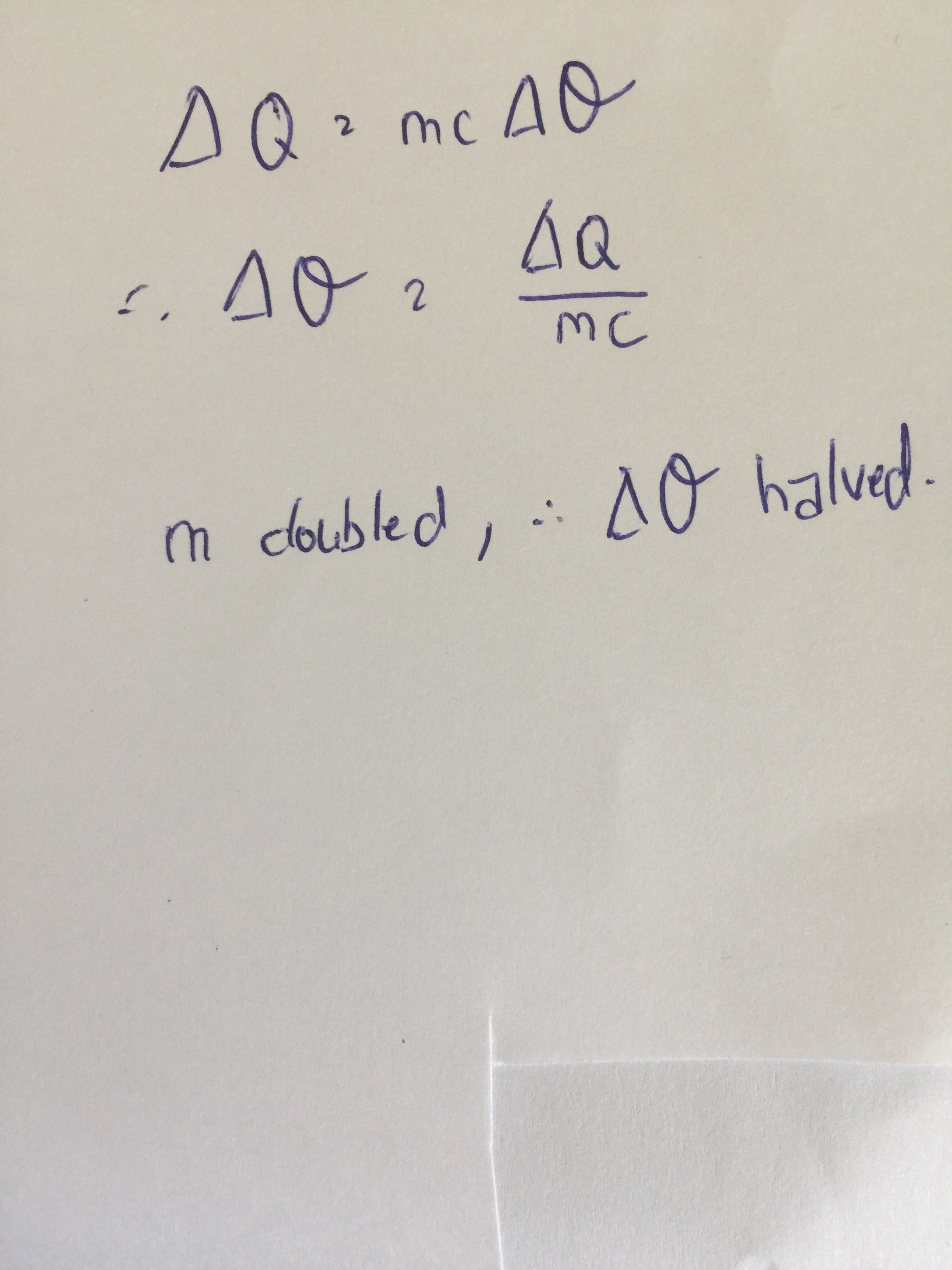Christmas MT's answer to Hi's Junior College 1 H2 Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

The number of moles of NaOH in both the first part and second part of the question are the same.
Therefore the number of moles of HCl required to neutralise it for both parts will also be the same.
Therefore the amount of heat produced during the reaction will be the same.
However, the volume of the solution doubled in the second part of the question.
Therefore the temperature increase is halved.
Therefore the number of moles of HCl required to neutralise it for both parts will also be the same.
Therefore the amount of heat produced during the reaction will be the same.
However, the volume of the solution doubled in the second part of the question.
Therefore the temperature increase is halved.
Date Posted:
7 years ago