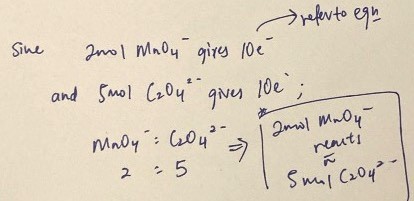Lim En Jie's answer to ok's Junior College 1 H2 Maths Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Since it’s MCQ don’t have to balance out equations, just observe the number of electrons used for each ion and then calculate their ratio.
In this case 2 mol of MnO4(-) will react with 5 mol of C2O4(2-).
Can’t think of any other reagents for titration but have to be careful; in this case 1 mol KMnO4 gives 1 mol MnO4(-) ion but may differ if another reagent used.
In this case 2 mol of MnO4(-) will react with 5 mol of C2O4(2-).
Can’t think of any other reagents for titration but have to be careful; in this case 1 mol KMnO4 gives 1 mol MnO4(-) ion but may differ if another reagent used.
Date Posted:
4 years ago