Eric Nicholas K's answer to Annela's Secondary 4 Chemistry Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
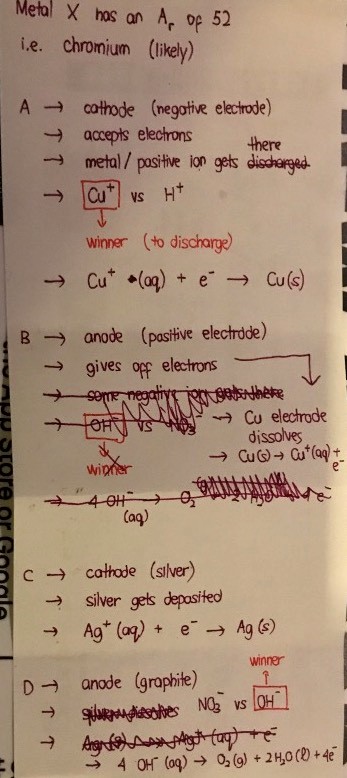
Good evening Annela! Here are my workings for electrodes A to D.
The identity of X should be chromium, Cr, which often has an ion charge of 3+.
I am suspecting that at electrode E, hydrogen is discharged instead of chromium even through the electrodes and the solution all are chromium based, because chromium is more reactive than hydrogen, and so hydrogen gets discharged first.
2 H+ (aq) + 2 e- —> H2 (g)
But at electrode F, the incoming electrons will first Attack the electrode itself than any of the anions in the solutions (containing OH- and another unknown anion). Hence, I suspect the chromium anode dissolves into the solution.
Cr (s) —> Cr3+ (aq) + 3 e-
I am not so sure about E and F though; these are just my thoughts.
The identity of X should be chromium, Cr, which often has an ion charge of 3+.
I am suspecting that at electrode E, hydrogen is discharged instead of chromium even through the electrodes and the solution all are chromium based, because chromium is more reactive than hydrogen, and so hydrogen gets discharged first.
2 H+ (aq) + 2 e- —> H2 (g)
But at electrode F, the incoming electrons will first Attack the electrode itself than any of the anions in the solutions (containing OH- and another unknown anion). Hence, I suspect the chromium anode dissolves into the solution.
Cr (s) —> Cr3+ (aq) + 3 e-
I am not so sure about E and F though; these are just my thoughts.
Date Posted:
4 years ago
Same thing, I am unable to help you much on this question.