Eric Nicholas K's answer to Arjun's Secondary 4 Chemistry India question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
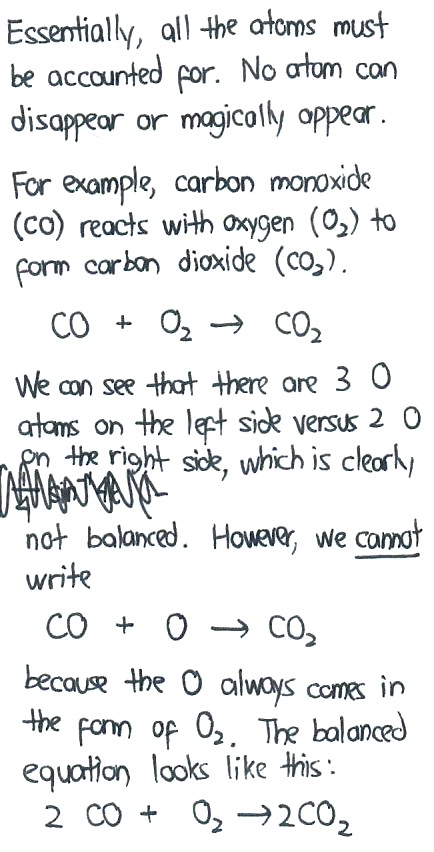
In my example, we can say that two molecules of carbon monoxide (CO) reacts with one molecule of oxygen (O2) to form two molecules of carbon dioxide (CO2).
Observe that we use integer values for the number of molecules (we cannot have HALF a molecule) while the entire equation is balanced. That is, there is an equal number of C on the left side and right side, and similarly, there is an equal number of O in the left side and right side.
Observe that we use integer values for the number of molecules (we cannot have HALF a molecule) while the entire equation is balanced. That is, there is an equal number of C on the left side and right side, and similarly, there is an equal number of O in the left side and right side.
Date Posted:
5 years ago