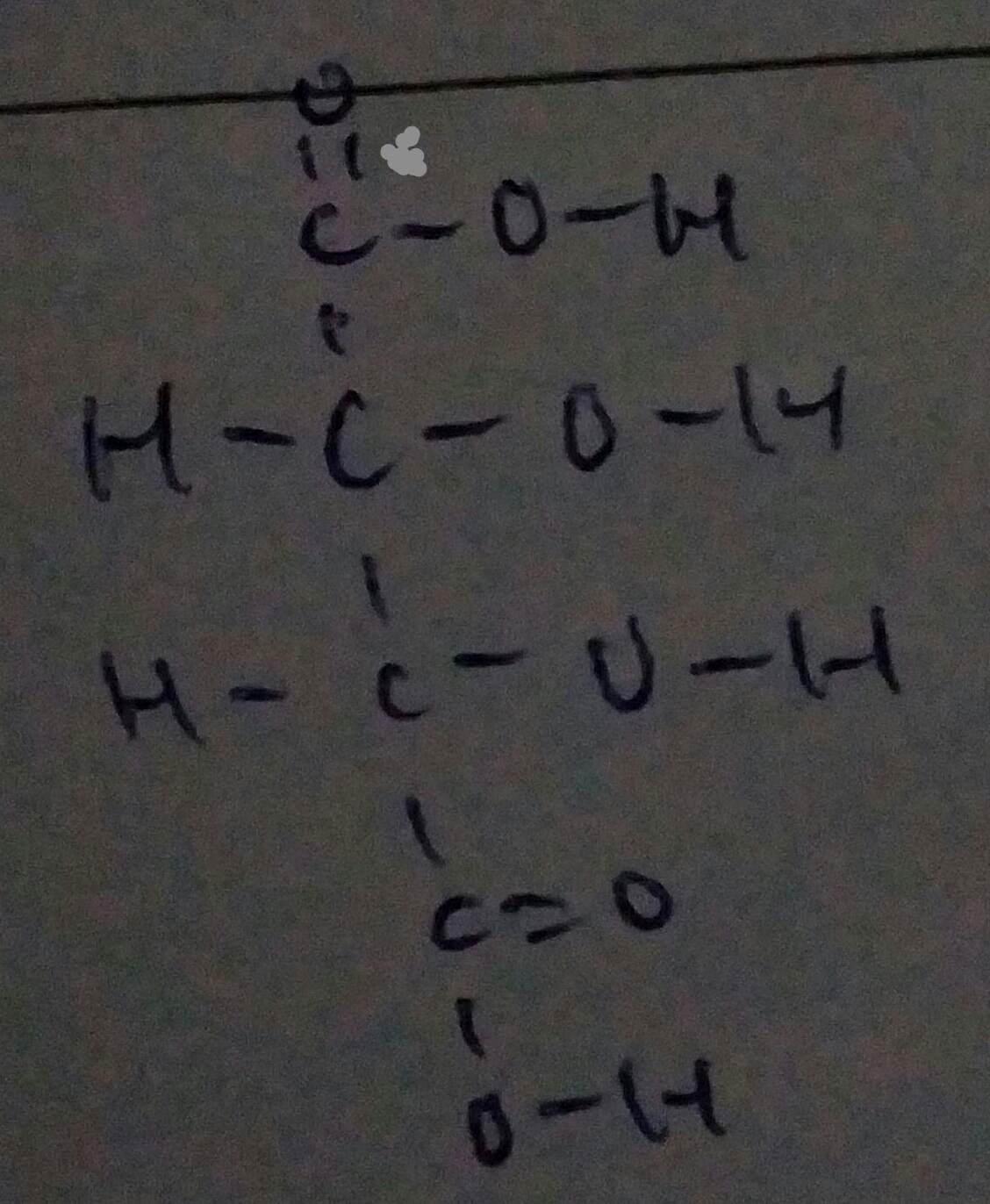Kee Ming Yuan's answer to mint's Secondary 3 Biology Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Carbon is in group 4 of elements so it can make 4 covalent bonds to be stable and every oxygen atom can have 2 covalent bonds as it is a group 6 element which lacks 2 electrons to achieve stability with 8 electrons in the valence electron shell, so the total no of bonds = no. of Oxygen atoms x 2 = 6 x 2 = 12.
Date Posted:
5 years ago
Question asks for total number of electrons, so the number of bonds have to be multiplied by 2 since 1 bond has 2 electrons.