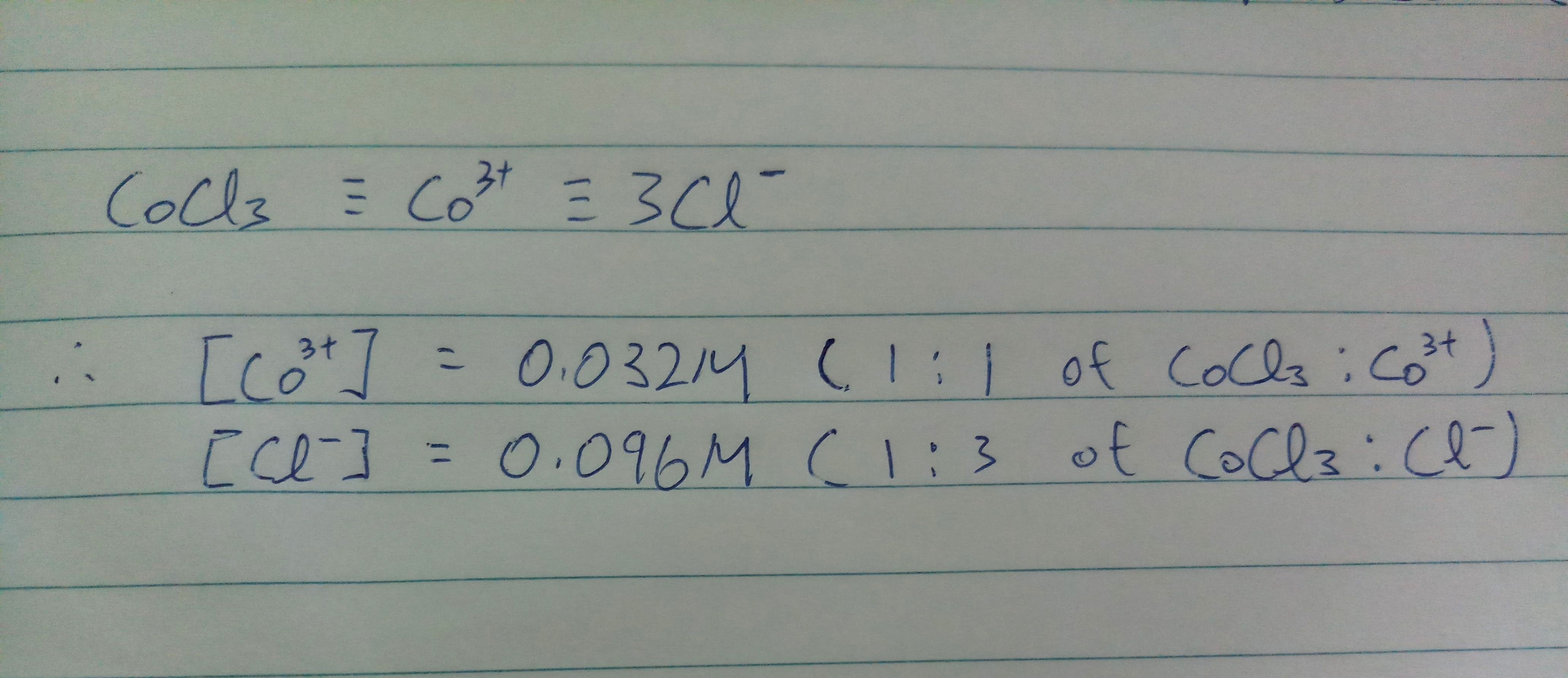Chee Kwok Wei's answer to Brittany's Junior College 2 H3 Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Assuming the volume is constant. Since CoCl3 and Co3+ have the same ratio, molar concentration of Co3+ will be the same a molar concentration of CoCl3.
Ratio of Cl-:CoCl3 is 3:1, molar concentration of Cl- is 3 times of the molar concentration of CoCl3
Ratio of Cl-:CoCl3 is 3:1, molar concentration of Cl- is 3 times of the molar concentration of CoCl3
Date Posted:
6 years ago