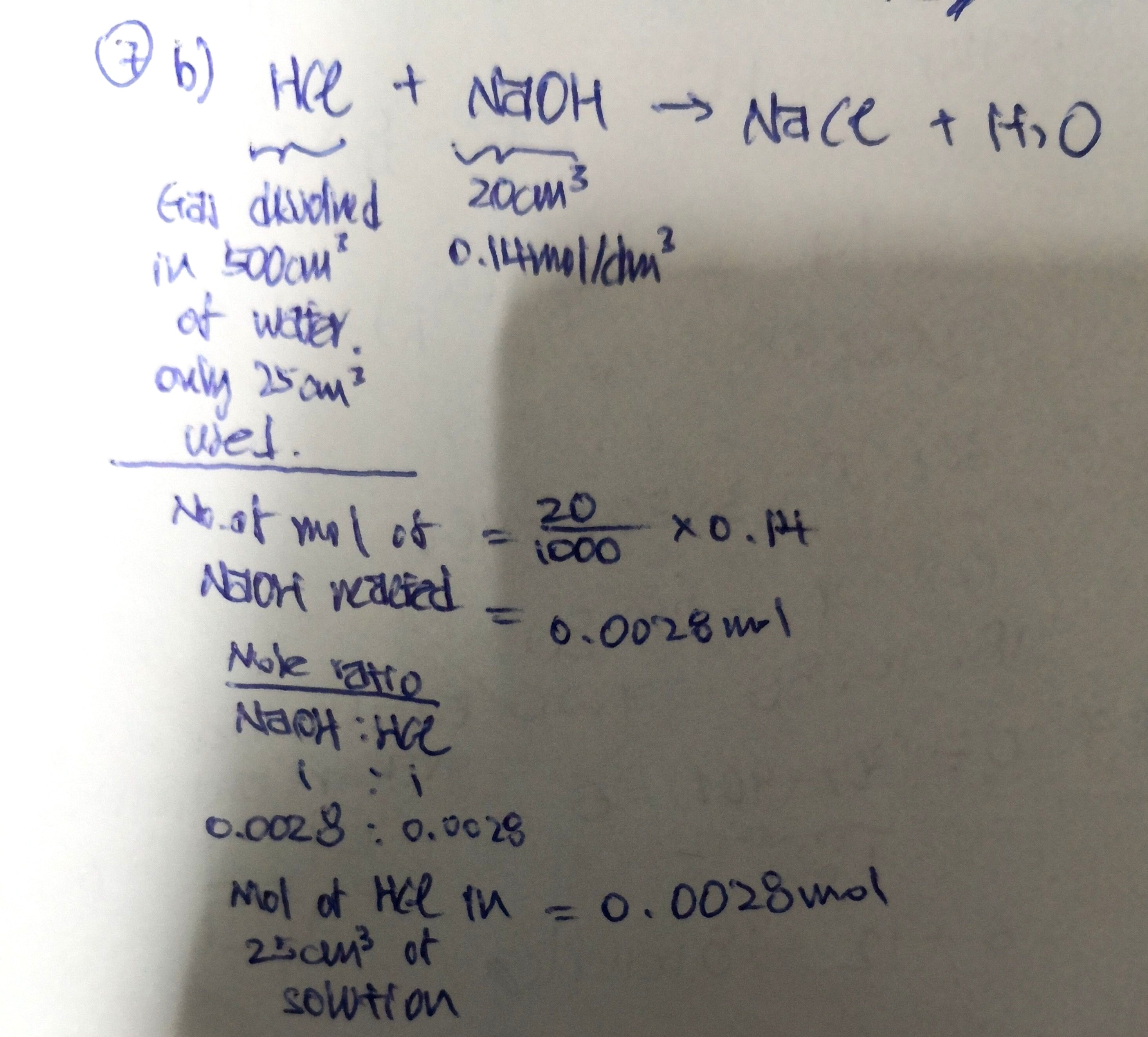Woon Kiat's answer to Skyler's Secondary 3 Chemistry question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
This is just part of the solution. Once you have the number of mol of HCl in 25cm3 of solution, scale it up to 500cm3 proportionally. That would be the amount dissolved originated from the gas.
From there, work out mol of XCl2. With mol and mass, you can find Mr of XCl2. You should know what to do next then...
From there, work out mol of XCl2. With mol and mass, you can find Mr of XCl2. You should know what to do next then...
Date Posted:
6 years ago
Why is it NaOH? shouldn’t it be KOH?
You are absolutely right. I made a careless mistake. Sorry.
Did you managed to complete the question?