Max's answer to Ru En Teh's Secondary 3 Chemistry question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

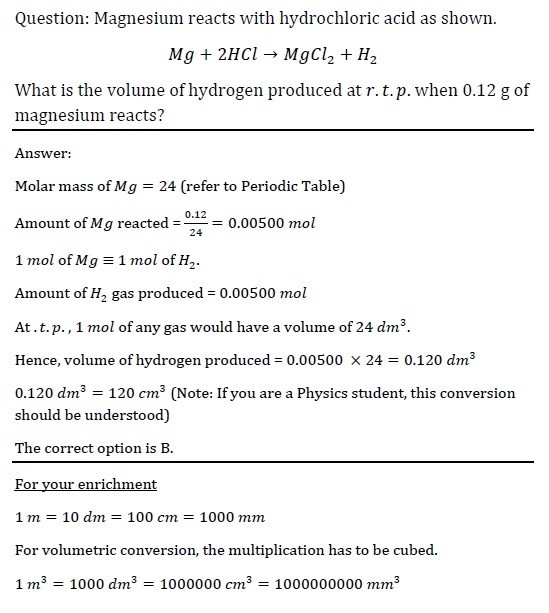
Key takeaways:
Volume of 1 mol of gas at room temperature and pressure is 24 dm3 / mol. (Versus 22.4 dm3 / mol at standard temperature and pressure)
Conversion methods for your enrichment are provided too!
Hope this helps!
Volume of 1 mol of gas at room temperature and pressure is 24 dm3 / mol. (Versus 22.4 dm3 / mol at standard temperature and pressure)
Conversion methods for your enrichment are provided too!
Hope this helps!
Date Posted:
8 years ago