Arnold K H Tan's answer to Ladybug's Secondary 4 Biology question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

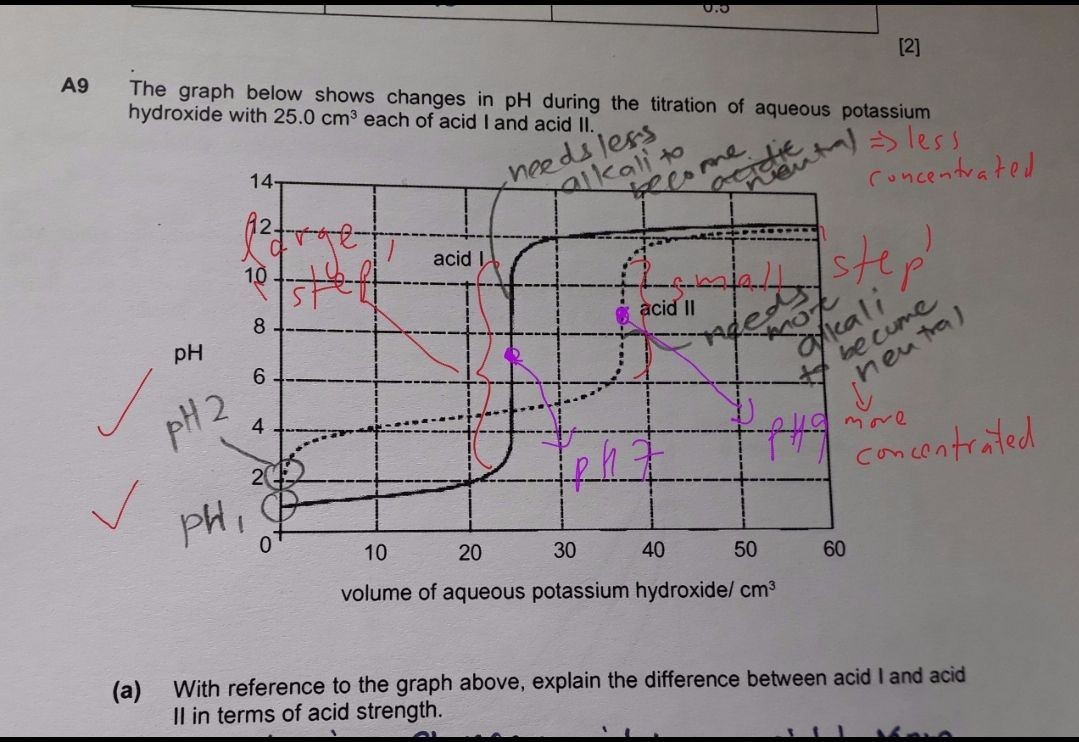
Acid 1 is a strong acid because the starting pH was pH1 and there is a large step during neutralisation, but of lower concentration compared to acid 2, as less alkali was needed for neutralisation. Acid 2 is a weak acid, as the starting pH was higher, but a smaller step during neitralisation point at pH 9 confirmed this. Concise answer: Acid 1 is a stronger acid that was neutralised at pH7 with a large pH 'step'. Less alkali used means the concentration of acid 1 was lower. Acid 2 was neutralised at pH9 with a smaller 'step', meaning acid 2 was a weak acid (but with a higher concentration). - words in brackets unneccessary... but add on if you have the answer space.
Date Posted:
6 years ago
Thank you! But what if they are asking to compare using concentration?
More alkali used => acid was more concentrated for the same 25cm3 acid used for the titration. The pH 'step' height differentiates between a strong and weak acid, as well as the neutralisation point. The starting pH is misleading as it may be a very dilute strong acid.
Thank you so much!