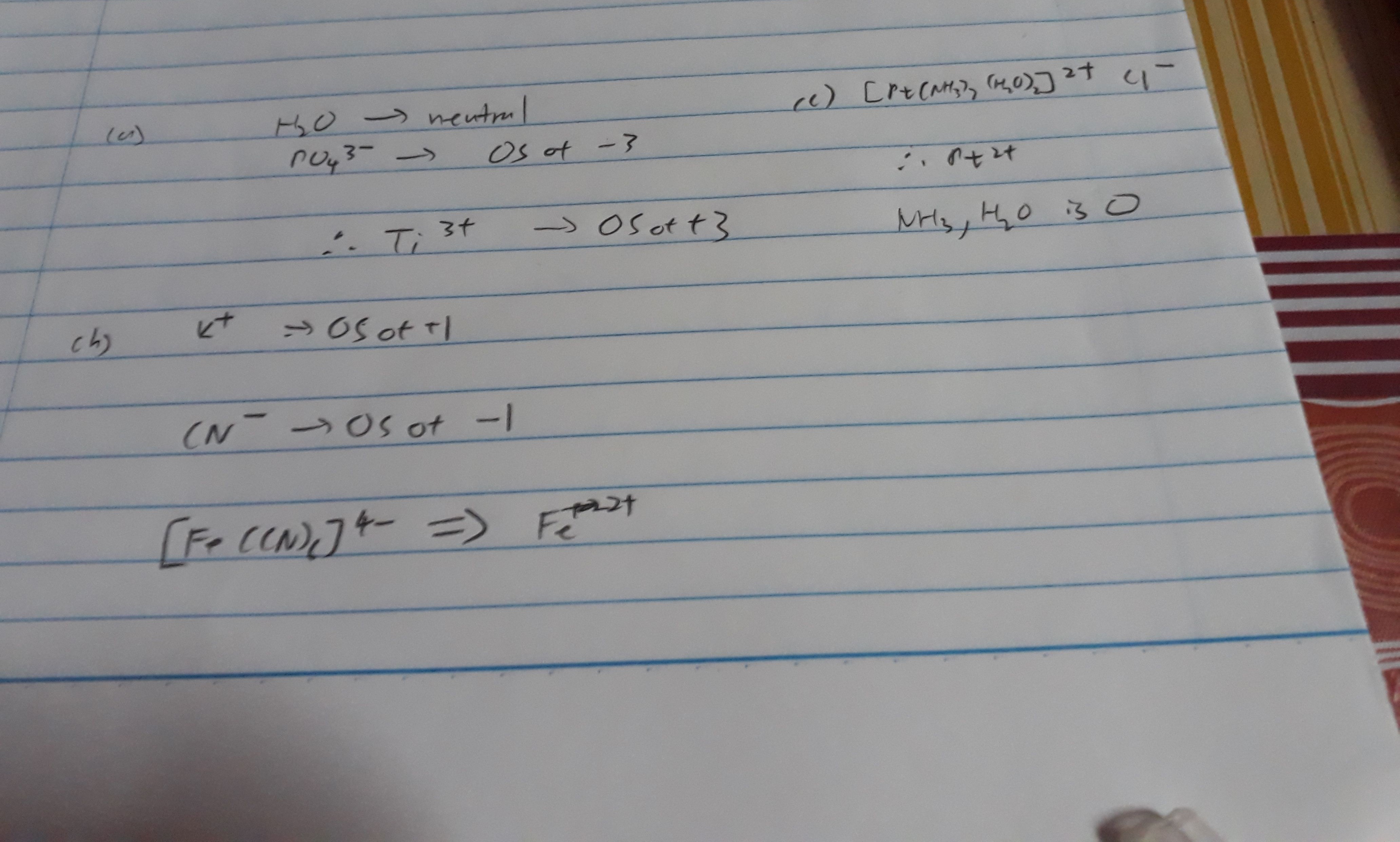Lee Wei Hao's answer to Chowder's Junior College 1 H3 Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Hope this helps
Date Posted:
6 years ago
Thank you but for Fe i got 4- . And can u help me with organic chemistry ? Please
Sure
But for fe, each CN is a -1 and total charge is -4. There are 6 CN which the total should be -6. Hence the reduction of 2 means that Fe is 2+
But i thought neutral compounds their oxidation state is 0?
And whr did u find the total charge as -4 ? There is for K which means +4 right but how T.T help me please please pleaseeee
K is group I. It is naturally +1. CN is naturally -1 as it is cyanide ion. CN is not neutral as c need to 4 electrons and n need only 3 electrons
Hi can u help me with principles of inorganic chemistry 2 ? Please?
If tertiary alkyl halide + weak nucleophile . Will it still undergo SN1 or it will undergo SN2?
If it is tertiary, always undergo sn1