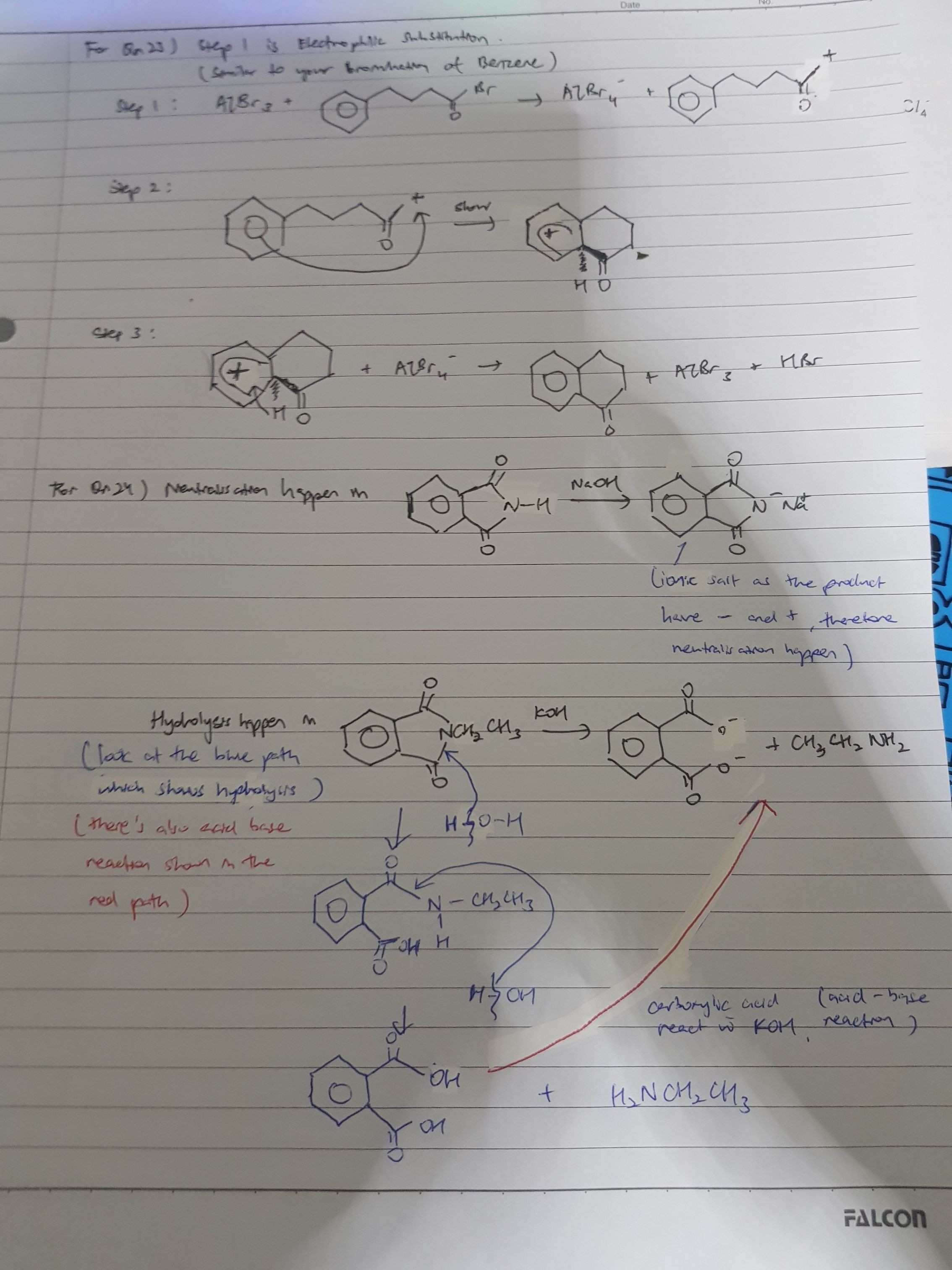
Tan Kiat Boon's answer to Jennifer's Junior College 2 H2 Maths question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Hope it helps! This is an application problem so hope you understand! Good luck n all the best! :D
Date Posted:
7 years ago
Thanks!!
For q24, i thought the N atom on the compund has lone pair available and hence an amine theres no neutralisation..
For q24, i thought the N atom on the compund has lone pair available and hence an amine theres no neutralisation..
Hey. The N atom have a lone pair but it's delocalised in to the 2 -C=O groups which are electron withdrawing. Thus, your N atom can accept electrons. Hope this helps! :D
Alright isee thanks!!