Tan Kiat Boon's answer to Jennifer's Junior College 2 H2 Maths question.
done
2 Upvotes
clear 0 Downvotes
Hope it helps! and it's Chemistry
All the best! :D
All the best! :D
Date Posted:
7 years ago
Thanks (:
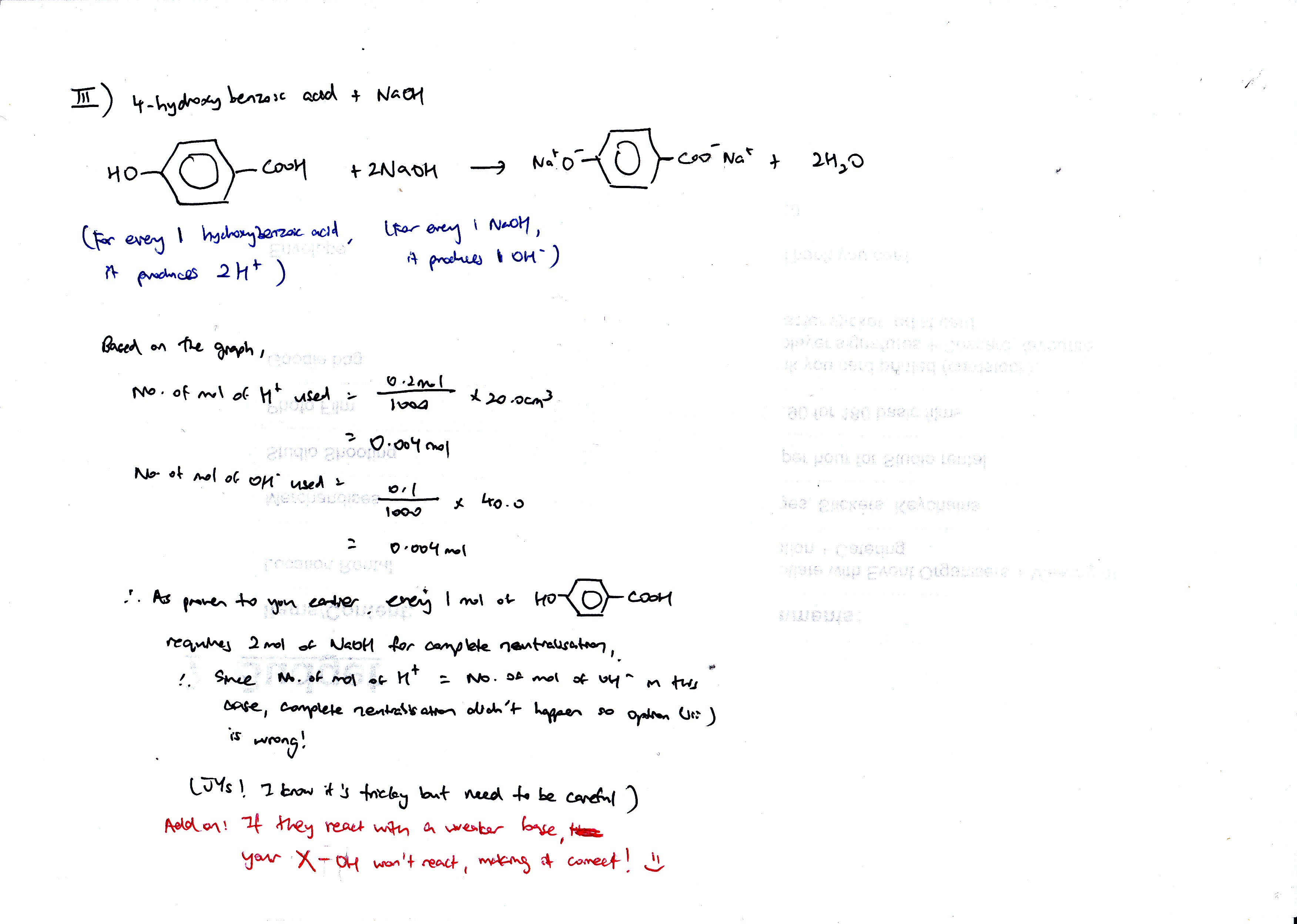
Why no complete neutralisation means thats not the ans btw?
As this experiment is a titration, only complete neutralization is possible for the PH to reach close to neutral once as it produces the salt only before it reached a high pH consisting of the NaOH. In this experiment, there is not enough NaOH when 40cm3 is used. Hence, the solution should still remain acidic and not start to get neutralise.