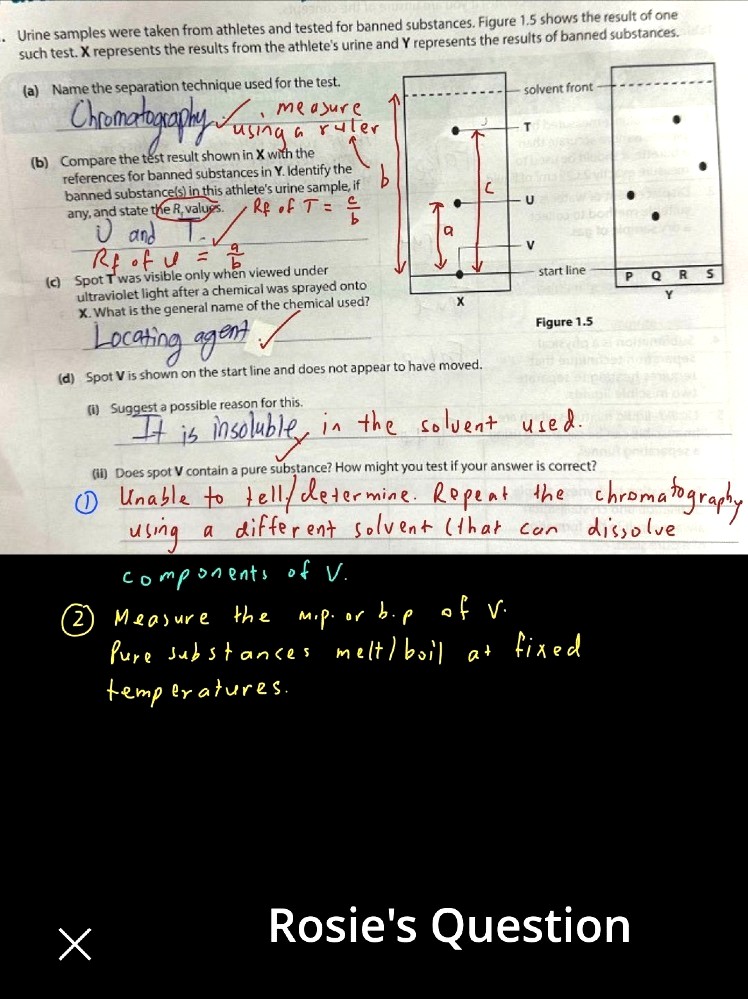
Arnold K H Tan's answer to Rosie's Secondary 3 E Maths Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

Need to be more detailed with you answers... as you're doing Pure Chem.
Note impure substances melt/boil over a range of temperature. Easiest test for purity, if your sample size is large enough...
Must also know the advantages of chromatography. Eg: one big advantage is that only a small sample amount is required.
Note impure substances melt/boil over a range of temperature. Easiest test for purity, if your sample size is large enough...
Must also know the advantages of chromatography. Eg: one big advantage is that only a small sample amount is required.
Date Posted:
1 year ago
oh I see but for the test for purity how does dissolving it in a solvent be able to tell whether it’s pure?
It does not. If you use a different solvent that dissolves the substance + run a new chromatography experiment, you can then observe if the substance separates into 2 or more components (= impure) on the chromatogram, or remains a single component.
ohh I see so it’s basically retesting to see if it will move when dissolving in a different solvent
Yes... but of course... can always add a little in each solvent to test if the sample can actually dissolve, before using the solvent in a chromatography experiment.