Kinson Gan's answer to V's Junior College 1 H2 Maths Singapore question.
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

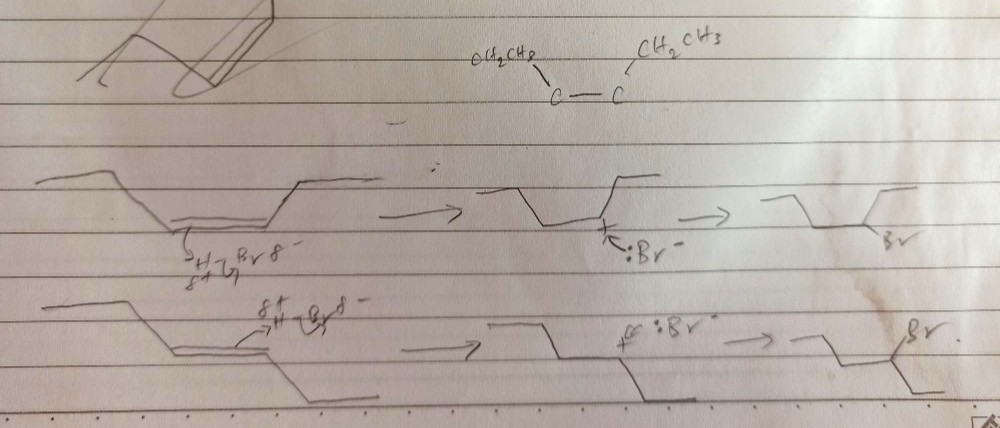
Top: cis-hex-3-ene
Bottom: trans-hex-3-ene
The 2 isomers react with Br- nucleophile using SN2 nucleophilic substitution mechanism as the carbocation (C with + charge) is surrounded by bulky hydrocarbon groups, leading to steric hindrance.
Since SN2 mechanism is where nucleophile attacks the carbocation from top or bottom of plane *with equal probability*, there are 2 equal proportion of products for each isomer of hex-3-ene.
Let me know if you need further clarification!
Bottom: trans-hex-3-ene
The 2 isomers react with Br- nucleophile using SN2 nucleophilic substitution mechanism as the carbocation (C with + charge) is surrounded by bulky hydrocarbon groups, leading to steric hindrance.
Since SN2 mechanism is where nucleophile attacks the carbocation from top or bottom of plane *with equal probability*, there are 2 equal proportion of products for each isomer of hex-3-ene.
Let me know if you need further clarification!
Date Posted:
3 years ago