Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | A Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

need help with this pure chem qn, pls explain too :)
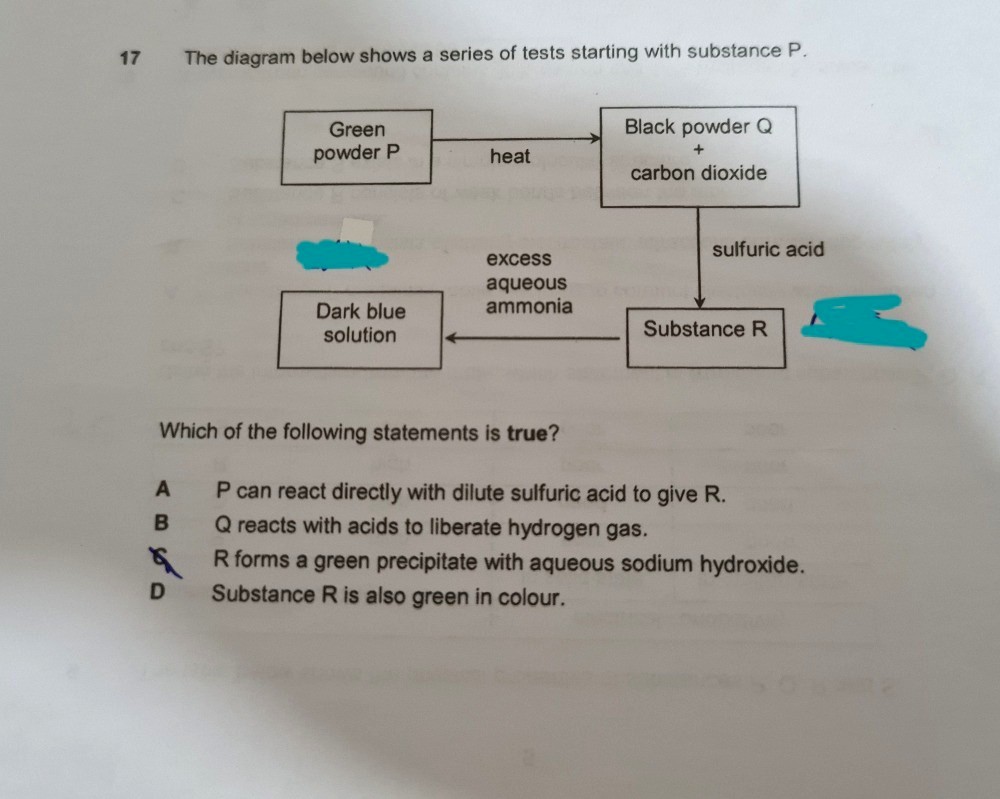
A lot of the copper (II) compounds are actually blue in colour, but there are some minor exceptions to this, one of which is copper (II) carbonate.
It's a matter of deduction thereafter to see whether the green powder is that of iron or that of copper.
You will notice that the dark blue solution upon the addition of excess aqueous ammonia is an indication that copper (II) ions are present in substance R (I assume you have learnt qualitative analysis). This, however, does not automatically mean that P must contain copper (II) ions but not zinc ions.
However, the addition of sulfuric acid to black powder Q (to obtain substance R) does not exactly bring about a "new colour change" at all. This tells us that black Q must have contained copper as part of its element constituents (where else can the copper in substance R come from?).
Black powder Q is obtained as a result of direct heating of the green powder P, so P must contain copper as part of its element constituents as well.
Because carbon dioxide is also generated in the heating process, the green powder P must contain some carbon as well, and this result is typical of carbonates.
So, a probable guess for the green powder P is copper (II) carbonate.
From here, we analyse the statements one by one.
"(A) P can react directly with dilute sulfuric acid to give R."
The above statement is equivalent to saying that copper (II) carbonate can react with dilute sulfuric acid to form copper (II) sulfate. This reaction can proceed as per regular acid-carbonate reactions, so this statement is true. Do note that pure copper metal, however, will not be able to react well with dilute sulfuric acid due to its lack of reactivity as referenced to the reactivity of hydrogen.
" (B) Q reacts with acids to liberate hydrogen gas."
The above statement is equivalent to saying that copper (II) oxide can react with acids to liberate hydrogen gas. The reaction of basic or amphoteric oxides with acids generally produces a metallic salt and water (the oxygen in water comes from the oxide) instead of hydrogen, so this statement is not true.
"(C) R forms a green precipitate with aqueous sodium hydroxide."
Since R is now known to be copper (II) sulfate, it should form a blue precipitate with aqueous sodium hydroxide.
I won't fully discount option (C) as I have not fully ruled out iron being present in the substance, but based on the information, we can expect copper to be present.
"(D) Substance R is also green in colour"
Similar to my discussion for option (C).
--------------------------------------------------
We are certain that option (A) is true, while the same cannot be said for options (C) and (D) while option (B) is certainly not true. As such, our best option for this question is option (A).
Copper is known to be blue, but some of its compounds are green in colour. In fact, in some scenarios the colour exhibited seems to be a hybrid of both colours (bluish-green), since some shades of blue and green look closely alike.
Fe²+ salts give a green ppt with dilute NaOH and is insoluble in excess. Likewise with aqueous NH3 (ammonia) .
Looking at the dark blue solution formed in the reaction scheme in the end, only Cu²+ salts will satisfy it (based on O level knowledge)
Well, some iron compounds are said to be “greenish-blue” at times, so it can get hard to distinguish between the two.
LockB, you will need to study the qualitative analysis results to answer the question.
See 1 Answer
I am a student myself as well
Just took my Os
In prelims i got 90/100 for chem
99/100 amath and 95/100 emath
So if u want u can email me
[email protected]
btw in an ionic compound, if the ratio of copper compared to the other element is smaller means theres a high chance that the colour of the ionic compound is not blue? like the example for CuCl2
But u just need to know that solution with Cu2+ generally is blue