Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
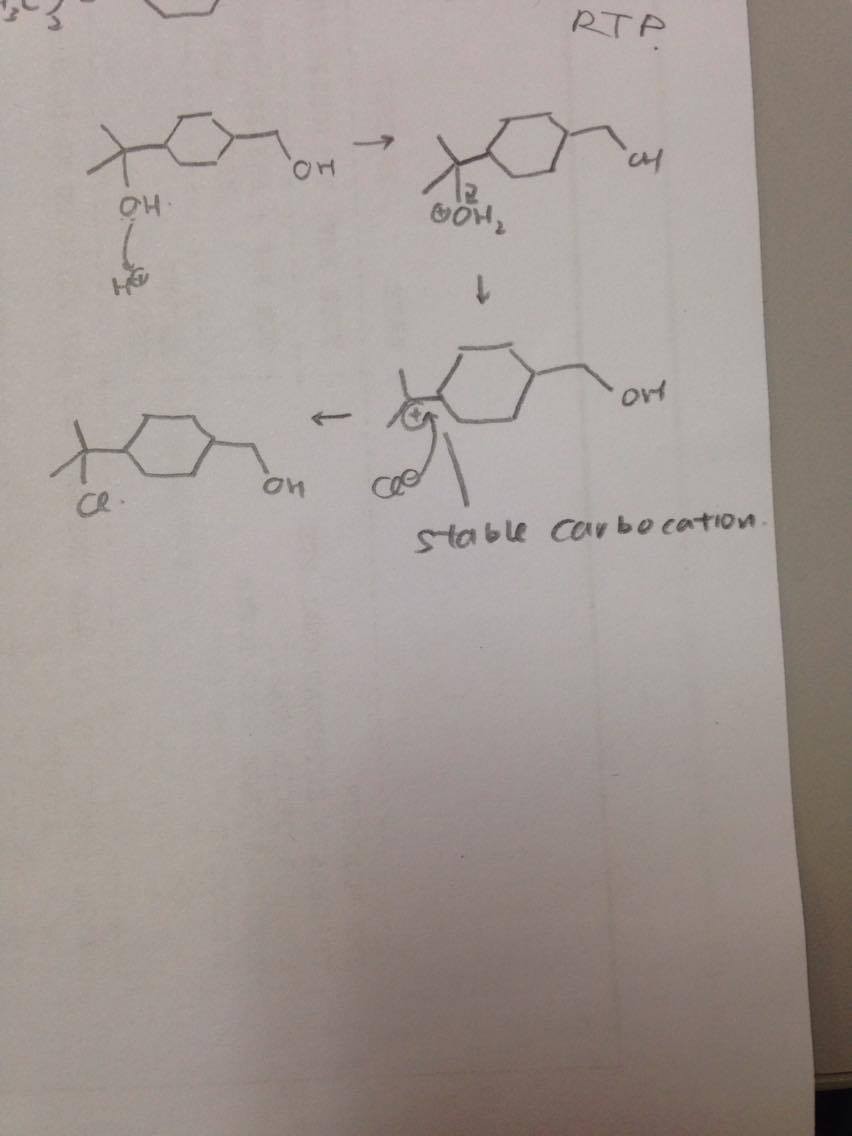
Acid catalysed SN1 substitution, tertiary alcohol will form the most stable carbocation.
Date Posted:
7 years ago
I dont understand . If theres 2 OH group the cl will only replace one? But how to know whr?
And how do i know whether its most stable? I thought only primary alcohol is stable T.T pls help me understand
Also doesnt the stability only tell us how fast the reaction is and not whr the CL is located ?
Regarding the stability of the molecule in the qn, you should consider the stability of the carbocation after the leaving group(OH) has left the molecule.
The reaction will occur preferably on a stable site, which is determined by the charge density of the carbocation formed. More positive -> less stable. Since alkyl groups are electrons donating, more alkyl groups -> carbocation less positive -> more stable site. Therefore the reaction will take place there
The reaction will occur preferably on a stable site, which is determined by the charge density of the carbocation formed. More positive -> less stable. Since alkyl groups are electrons donating, more alkyl groups -> carbocation less positive -> more stable site. Therefore the reaction will take place there
As for the position of the substitution. Both OH sites will eventually get substituted given that there is excess of the reagent. However the tertiary alcohol forms a more stable carbocation which is a more preferred site over the primary alcohol site therefore it will get substituted first before the reaction moves on to the primary alcohol. And since the question mentions the MAIN product, the substituted tertiary alcohol will be the ans. Just a side note, the reagent used is called the Lucas reagent, it is used to differentiate the different types of alcohols based on reaction time. Go google!
looking back at your question, there is only 1 HCl in the question, that means you only have 1 Cl- available for substitution, ZnCl2 is the catalyst for this reaction, so the 2Cl- in ZnCl2 are not available for substitution. So for this question, although there is 2 OH group, only 1 OH will be substituted by 1 Cl-. (note the stoichiometry)
How to choose the which OH to be substituted? Always remember the stability goes by tert > sec > primary. Since this is a SN1 substitution reaction, rate does not matter but stability of carbocation matters more, rate matters more in SN2 reaction.
Why tert>sec>primary?
refer to my drawing, a tert alcohol will form a tert carbocation, the plus charge will be stabilised by more electron density surrounding the plus charge, hence it is a more stable carbocation, make it stays stable for longer, long enough for Cl- to attack the carbocation.
How to choose the which OH to be substituted? Always remember the stability goes by tert > sec > primary. Since this is a SN1 substitution reaction, rate does not matter but stability of carbocation matters more, rate matters more in SN2 reaction.
Why tert>sec>primary?
refer to my drawing, a tert alcohol will form a tert carbocation, the plus charge will be stabilised by more electron density surrounding the plus charge, hence it is a more stable carbocation, make it stays stable for longer, long enough for Cl- to attack the carbocation.
What does SN2 means. I have another question but i cant seem to post the pic here :(
The stability can also tell us the product form?
SN2 means nucleophilic substitution reaction with a bimolecular rate law. Which means rate = k[nucleophile][reactant molecule with leaving group]
Cn help me with chem pls