Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Hi, this is the answer to the question.
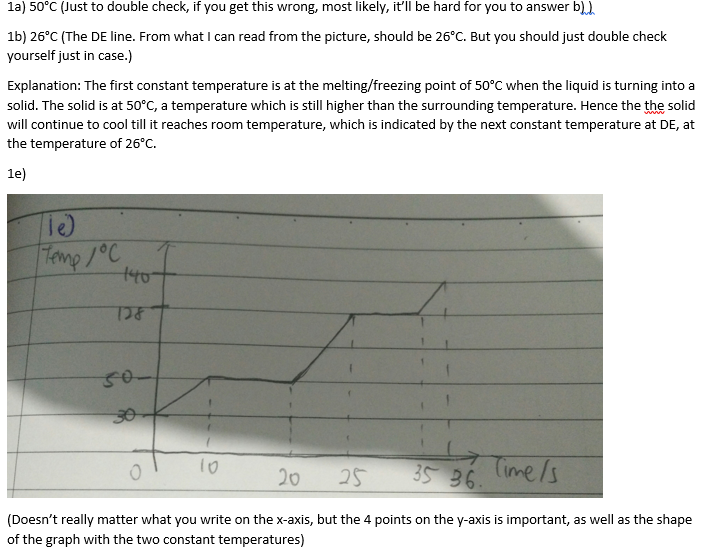
Take note that the question states that the liquid is left to cool in the air, so it's not being put in the freezer or anything, so the FINAL temperature (DE) that the liquid is at should be the room temperature.
The one thing which may be confusing is the two constant temperature lines. The first one is the melting/freezing point at BC. But because that temperature is still higher than the surrounding temperature, that's why the temperature after BC will continue to drop until it reaches DE, which is the room temperature.
For the sketching, take note that from 30 to 140, there will be two constant lines. One at 50 (melting pt) when the solid is melting into liquid, the second at 128(boiling pt) when the liquid is turning into gaseous state. After all the liquid has turned into gaseous state, the temperature should continue increasing all the way to 140.
Hope this helps. :)
Take note that the question states that the liquid is left to cool in the air, so it's not being put in the freezer or anything, so the FINAL temperature (DE) that the liquid is at should be the room temperature.
The one thing which may be confusing is the two constant temperature lines. The first one is the melting/freezing point at BC. But because that temperature is still higher than the surrounding temperature, that's why the temperature after BC will continue to drop until it reaches DE, which is the room temperature.
For the sketching, take note that from 30 to 140, there will be two constant lines. One at 50 (melting pt) when the solid is melting into liquid, the second at 128(boiling pt) when the liquid is turning into gaseous state. After all the liquid has turned into gaseous state, the temperature should continue increasing all the way to 140.
Hope this helps. :)
Date Posted:
8 years ago