Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
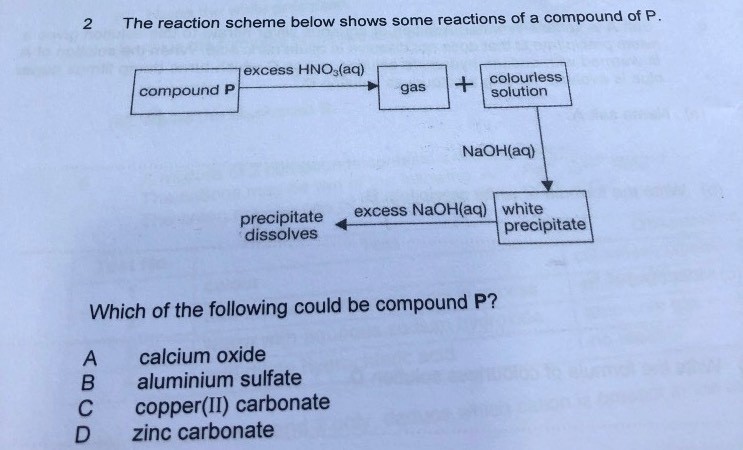
Answer is B. Aluminium Sulfate
Aluminium salt precipitate dissolves in excess NaOH.
Calcium salt precipitate does not.
Aluminium salt precipitate dissolves in excess NaOH.
Calcium salt precipitate does not.
I’m pretty sure it’s zinc carbonate.
Aluminium sulfate is very close, except that I doubt a gas would be formed.
What’s more, I am doubtful that aluminium sulfate (a salt) will even react with nitric acid, since the “would-be products” would have been aqueous as well.
Nancy, have you learnt the cationic and anionic tests? The one which gives precipitates upon addition with NaOH etc. And have you learnt reaction of acids? You will need these knowledge to solve this question.
Aluminium sulfate is very close, except that I doubt a gas would be formed.
What’s more, I am doubtful that aluminium sulfate (a salt) will even react with nitric acid, since the “would-be products” would have been aqueous as well.
Nancy, have you learnt the cationic and anionic tests? The one which gives precipitates upon addition with NaOH etc. And have you learnt reaction of acids? You will need these knowledge to solve this question.
^
The answer is zinc carbonate.
A gas is produced after P is added with nitric acid, look at the first two options. First option, calcium oxide reacts with nitric acid to form aqueous calcium nitrate and water. This is your neutralisation reaction. Option 2, in your syllabus, you have not learnt any salts that can react with acids to give off a gas. That option is out. Let's look at options 3 and 4. One is copper(II) carbonate and other is zinc carbonate. Refer back to QA chart, upon addition of NaOH, copper(II) salts will give a blue ppt whereas zinc salts will give a white ppt. Upon excess NaOH, blue ppt dissolves to give a dark blue solution and white ppt dissolves into a colourless solution. This eliminates the 3rd option since we are looking for a white ppt that dissolves into colourless solution upon excess NaOH
A gas is produced after P is added with nitric acid, look at the first two options. First option, calcium oxide reacts with nitric acid to form aqueous calcium nitrate and water. This is your neutralisation reaction. Option 2, in your syllabus, you have not learnt any salts that can react with acids to give off a gas. That option is out. Let's look at options 3 and 4. One is copper(II) carbonate and other is zinc carbonate. Refer back to QA chart, upon addition of NaOH, copper(II) salts will give a blue ppt whereas zinc salts will give a white ppt. Upon excess NaOH, blue ppt dissolves to give a dark blue solution and white ppt dissolves into a colourless solution. This eliminates the 3rd option since we are looking for a white ppt that dissolves into colourless solution upon excess NaOH