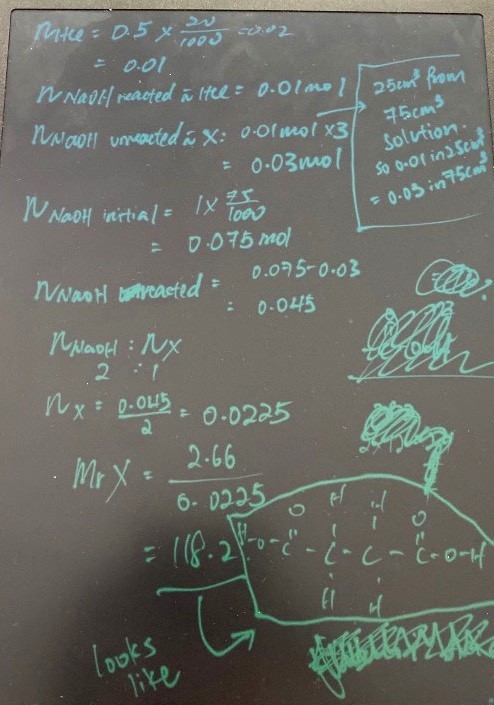Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 1 | H2 Maths
2 Answers Below
Anyone can contribute an answer, even non-tutors.

Good morning:) Can someone please help me with (a)? I don’t know which information to use in this question to get to the answer.. I found the original moles of NaOH and the amount of HCl used but i cant carry on from there. Thank you for helping !
See 2 Answers
Firstly, we can deduce that X has (-COOH) functional group because the question states it is an organic acid.
X is an acid of unknown concentration so by using a fixed volume of X against an excess of known concentration/volume of NaOH, you will get something as follows;
RXN1: NaOH (excess) + X = H2O + Na2X + NaOH (remaining unreacted product)
*since an excess of NaOH was used, you cannot be sure how much of it reacted with compound X which is why;*
The second titration is required using HCl — to determine the amount of NaOH that is unreacted in the RXN1 shown as follows;
RXN2: NaOH (remaining unreacted from RXN1 product) + HCl = H2O + NaCl
From RXN2, you now know the remaining Mol NaOH (unreacted) in RXN1.
You can then use the initial Mol NaOH (excess) - Mol NaOH (unreacted) to get Mol NaOH (reacted).
Getting Mol NaOH (reacted) means you can use ratio of
Mol X (dibasic) : Mol NaOH (reacted)
1 : 2
To find the Mol X, of which divided from Mass gives you Mr.
* You use a fixed mass of 2.66g of X not fixed volume.
* Number mol NaOH reacted with HCl is 1/3 of NaOH unreacted because you took 25cm3 from a volume of 75 cm3.
Also to add on:
You use a fixed conc&volume of HCl in RXN2 to neutralise the unknown number mol NaOH (unreacted product) in RXN1.
This is so you can calculate the number mol of HCl used = number mol of NaOH unreacted in RXN1.
Having number mol of NaOH (unreacted) and number mol NaOH (excess) means you can calculate number mol NaOH (reacted with X).