Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | E Maths
2 Answers Below
Anyone can contribute an answer, even non-tutors.

ITS SCIENCE CHEM YALL IGNORE THE SUBJ.
Do I have enough Na2SO4 to react fully with all the available Pb(NO3)2?
Do I have enough Pb(NO3)2 to react fully with all the available Na2SO4?
This is based on the stoichiometric ratio 1 : 1.
Think of baking.
Suppose a classroom goes like this.
1 table + 4 chairs = 1 seating area
(and the ratio remains fixed).
If I have 4 tables and 10 chairs to be placed in the fixed ratio,
- do I have enough tables?
- do I have enough chairs
- how many groups of seating area can I have at most?
The idea for this question is very similar.
See 2 Answers
Imagine you are an athlete training up for a championship. You are training with your coach.
Would you want your performance level to stay the same at the end of the months-long training period? Or would you want your performance to improve drastically to help you earn a better result?
For most athletes, they will know the obvious.
Now, in this ionic equation, we would like to see a change happening in the system. This is seen by a change in physical states (solid, liquid, aqueous, gaseous).
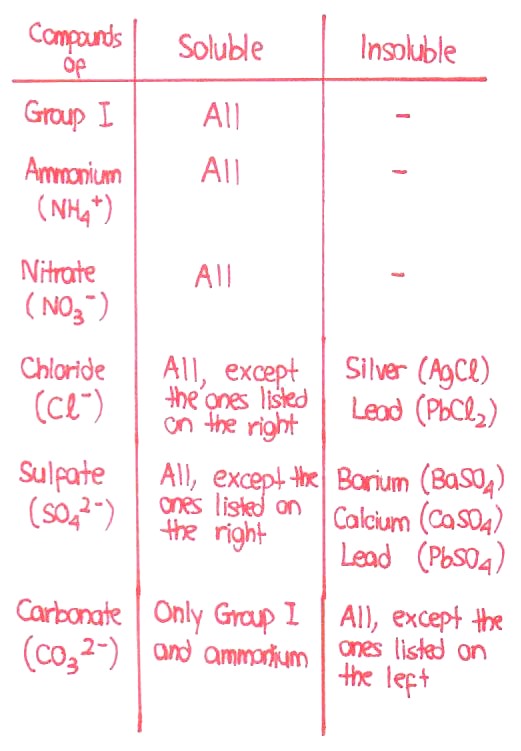
Before you even attempt to write down an ionic equation, you will need to know the solubilities of your compounds, which I have attached in the first post.
Hang on for my next part.
Now, here the two reactants, sodium sulfate and lead nitrate are soluble in water. They don't react with each other as solids, so they have to be in a dissolved form, usually in water. We call this aqueous. Similarly, one of the compounds formed. sodium nitrate, is soluble in water, and is thus in an aqueous state since the water of reaction is still present.
(If you react hydrochloric acid with sodium hydroxide to form sodium chloride, the sodium chloride won't pop out as a white solid correct?)
So, here's the thing. When dissolved in water, these soluble compounds will ALWAYS split into their respective ions, because the water of dissolution overcomes the ionic lattice structure of the ionic compound. In contrast, insoluble compounds cannot be split into their respective ions as the water of dissolution cannot break the ionic lattice structure.
As such,
- sodium sulfate exists as sodium ions (Na +) and sulfate (SO4 2-) ions in water due to its solubility
- lead nitrate exists as lead (Pb 2+) ions and nitrate (NO3 -) ions in water due to its solubilty
- sodium nitrate exists as sodium (Na +) and nitrate (NO3 -) ions in water due to its solubility
- lead sulfate exists as a plain solid itself without breaking down into ions, due to its lack of solubility
Wait for my next post.
-----------------------------------------------
BEFORE
The species present before the reaction are
- Na + (aq),
- SO4 2- (aq)
- Pb 2+ (aq)
- NO3 - (aq)
-----------------------------------------------
AFTER
The species present after the reaction are
- PbSO4 (s) (a full solid, no more ions)
- Na + (aq) (since the resultant compound with NO3- is still aq)
- NO3 - (aq) (since the resultant compound with Na+ is still aq)
-----------------------------------------------
It's kind of like dancing. Suppose I am in a dance room with 3 other people (as an example).
The four of us started out without partners (aq). The objective at the end is to link up with someone else for the dance.
I start out as Na +. At the end of the dance session, I am still aq, meaning I have not found a suitable partner for the dance. Mission failed.
A second person starts out as NO3 -. At the end of the dance session, the person is still aq as well, so the person also fails the mission.
We are both out of the ionic equation.
The other two people, Pb 2+ and SO4 2-, manage to find their dance partners (i.e. each other), so it's a success (they no longer stay as aq and become s).
These "successes" give rise to their presence in the ionic equation.
-----------------------------------------------
So in summary,
- Pb 2 + and SO4 2- link up together and are therefore in the ionic equation
- Na + and NO3 - remain as Na + and NO3 - even at the end, so they are NOT in the ionic equation
-----------------------------------------------
Therefore, the ionic equation is
Pb 2+ (aq) + SO4 2- (aq) --> PbSO4 (s)
-----------------------------------------------
Let me know if you need more explanation or a second example.