Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 1 | H2 Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

h2 chem ! help me pls thanks !
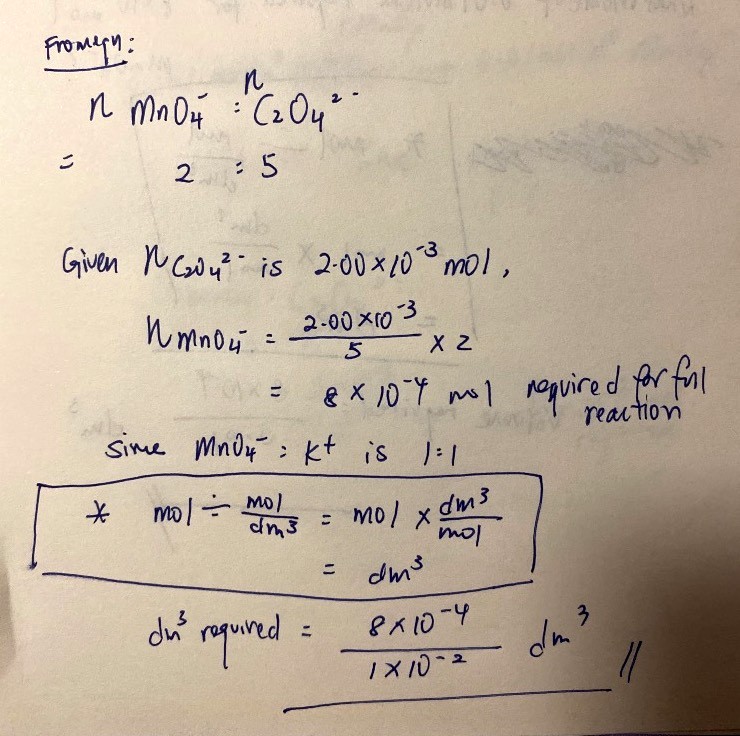
Number of mol of KMnO4 required
= 2.00 x 10-³ mol ÷ 5 x 2
= 8.00 x 10-⁴ mol
Volume of KMnO4 required
= 8.00 x 10-⁴ mol ÷ 0.0100 mol/dm³
= 8.00 x 10-² dm³
See 1 Answer