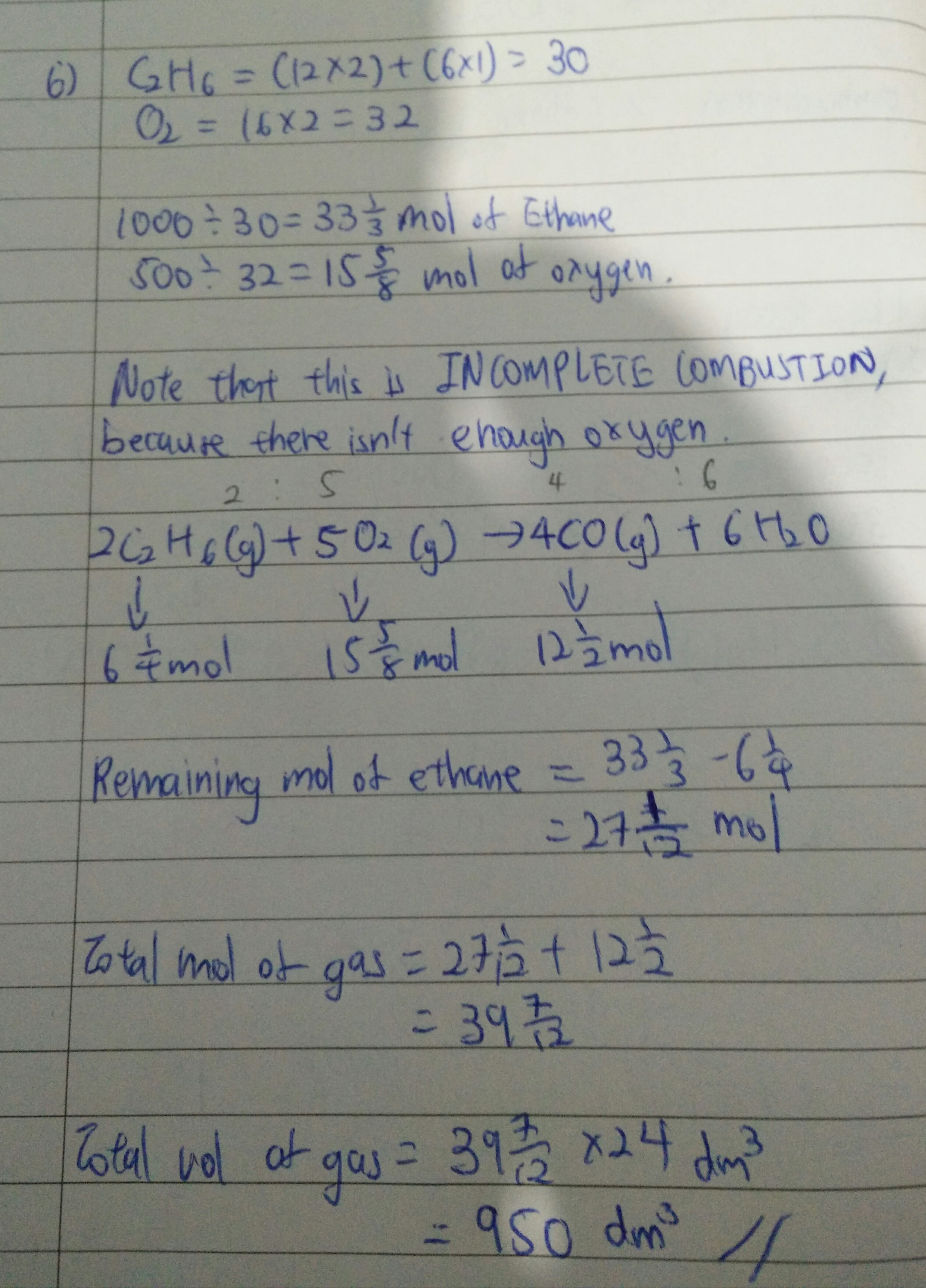Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Hi, you got 907 dm3 because you assumed that this will be a complete combustion, so you used the wrong mol ratio. In actual fact, this is actually an INCOMPLETE combustion, because you don't have enough mols of oxygen. For a complete combustion, you need to have at least 116 2/3 mols of oxygen to react with 33 1/3 mols of ethane .
So for INCOMPLETE combustion, the gas produced will not be CO2, but CO (carbon monoxide). The mol ratio will also be different.
If you managed to get 907, your strategy and calculations of mol and vol are theoretically sound. Just that you have to remember that there are two kinds of combustion. Complete and Incomplete in the future. Don't always assume that it's going to be complete combustion.
Hope this helps. If you still don't understand, I think you can try commenting in the comment section and I'll try to help. :)
So for INCOMPLETE combustion, the gas produced will not be CO2, but CO (carbon monoxide). The mol ratio will also be different.
If you managed to get 907, your strategy and calculations of mol and vol are theoretically sound. Just that you have to remember that there are two kinds of combustion. Complete and Incomplete in the future. Don't always assume that it's going to be complete combustion.
Hope this helps. If you still don't understand, I think you can try commenting in the comment section and I'll try to help. :)
Date Posted:
7 years ago