Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

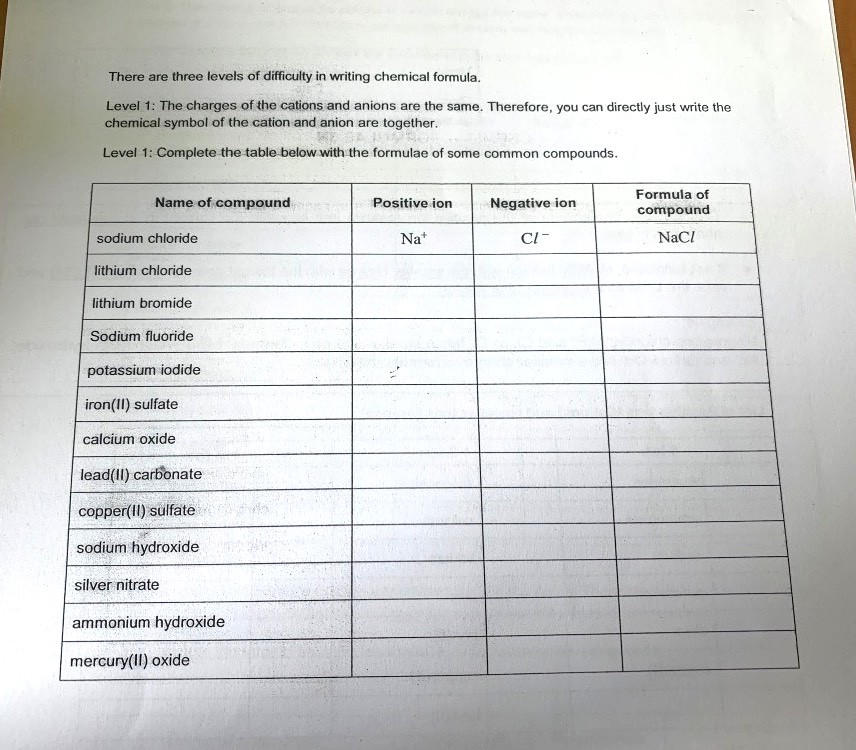
Question
secondary 3 | A Maths
3 Answers Below
Anyone can contribute an answer, even non-tutors.

this is secondary 3 chemistry , sorry but i doesn't have the premium thingy so :/ rly hope yall can help me bcus idk how to do , thank you ! :-)
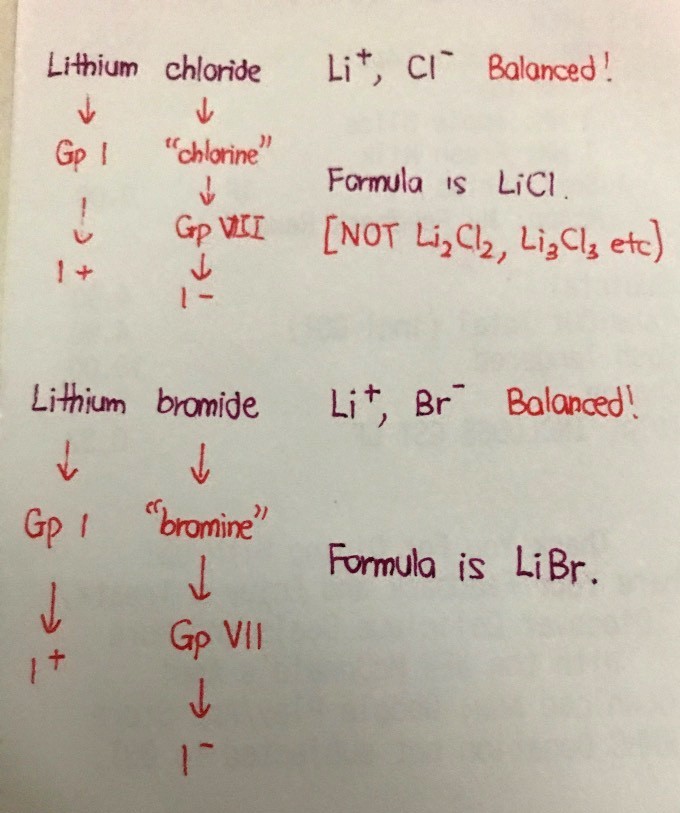
lithium chloride:
lithium is in grp I, same as sodium & potassium.
symbol for lithium is Li
just like sodium (Na) in grp I,
the +ve ion for lithium chloride is Li⁺
fluoride, chloride, bromide & iodide are known as halides. they r in grp VII & grp VII elements are known as halogens.
grp VII is next to grp VIII or grp 0, so halides r more likely to be -ve ions.
since VII is 1 less than VIII, the charge is -1.
hence chloride is Cl⁻.
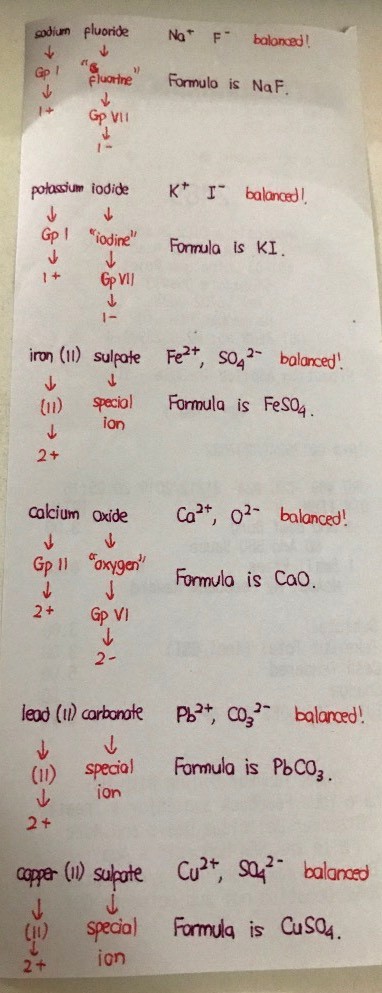
from above, u can figure out the +ve & -ve ions for lithium bromide, sodium fluoride & potassium iodide
iron(II) sulfate:
iron ions can be +2 charged or +3 charged (bcos it is a transition metal)
as the name above implied, we know this is iron(II) or the ion is +2 charged
hence, Fe²⁺
sulfate ion is a standard SO₄²⁻.
this is knowledge.
there is a why for it, may be TMI for u, but not necessary for u at Sec 3.
calcium oxide:
calcium is in grp II.
its ion is +ve charge.
hence, Ca²⁺
oxygen is in grp VI
its ion is -ve charge.
since VI is 2 less than VIII
the -ve ion for oxide is O²⁻
lead(II) carbonate:
lead ions can be +2 charged or +4 charged (bcos it is a transition metal)
as the name above implied, we know this is lead(II) or the ion is +2 charged
hence, Pb²⁺
carbonate ion is a standard CO₃²⁻.
this is knowledge.
there is a why for it, may be TMI for u, but not necessary for u at Sec 3.
copper(II) sulfate:
copper ions can be +1 charged or +2 charged (bcos it is a transition metal)
as the name above implied, we know this is copper(II) or the ion is +2 charged
hence, Cu²⁺
u know sulfate above
sodium hydroxide:
sodium is in grp I,
what do u think is the +ve ion ?
hydroxide ion is a standard OH⁻.
this is knowledge.
there is a why for it, may be TMI for u, but not necessary for u at Sec 3.
silver nitrate:
silver is more complex bcos it is a transition metal and amphoteric.
it can have an oxidation state of -2, -1, +1, +2 or +3.
u can google abt it but it may be TMI for u.
impt thing is +1 charge is the most stable for silver.
hence, Ag⁺
nitrate ion is a standard NO₃⁻.
this is knowledge.
there is a why for it, may be TMI for u, but not necessary for u at Sec 3.
ammonium hydroxide:
ammonium hydroxide is one of the alkalis u can find ur chem lab, alongside sodium hydroxide & potassium hydroxide.
so ammonium hydroxide is quite similar to sodium hydroxide.
ammonium ion is a standard NH₄⁺.
this is knowledge.
there is a why for it, may be TMI for u, but not necessary for u at Sec 3.
u know hydroxide ion above
mercury symbol is Hg
what do u think mercury(II) oxide contains?
Let me know if you need me to pen this down on paper. It's a very similar approach to your previous post (probably coming from the same worksheet).
" mercury ( II ) oxide contains Hg^2+ , is it ? "
gd job!
u r getting the hang of it
.
yup , is it possible for u to pen it down & show me ? thank you so much !
See 3 Answers