Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

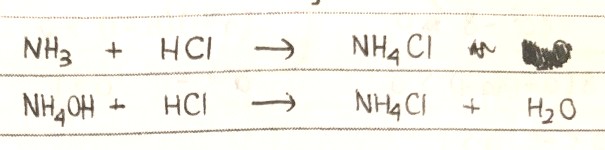
help with this eqn pls
NH3 is a basic gas, HCl is an acid, and the direct compound formed is NH4Cl.
It would not be appropriate to include water as the product in the equation as this leads to an imbalance of atoms on both sides (since oxygen “magically” appears out of nowhere).
But dissolve NH3 in water to get the alkaline NH4OH and your product will also contain water (think of the ions present in these compounds).
This is actually a fairly common reaction of 2 gases. Both NH3 and HCl are gases here.
So HCl is not in acid form i.e not dissolved in water/no water present.
So the only product is NH4Cl
Maybe the teacher is talking about aqueous ammonia and hydrochloric acid. But that would result in 1 product as well.
The problem is no state symbols are given in the question.
Aqueous ammonia is basically ammonium hydroxide.
I.e doesn't exist as a NH4OH formula unit. In the past for my time we were instructed not to write NH4OH.
I would offer two equations for this question :
NH3(g) + HCl (g) → NH4Cl (s)
NH3(aq) + HCl (aq) → NH4Cl (aq)
I could not remember whether my syllabus required me to write NH4OH or avoid writing it.
The correct equilibrium equation is
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH- (aq)
The two ions are detached and not bound to each other as suggested in NH4OH
I.e
NH3(g) → NH3(aq)
See 1 Answer