Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

chemistry question!!
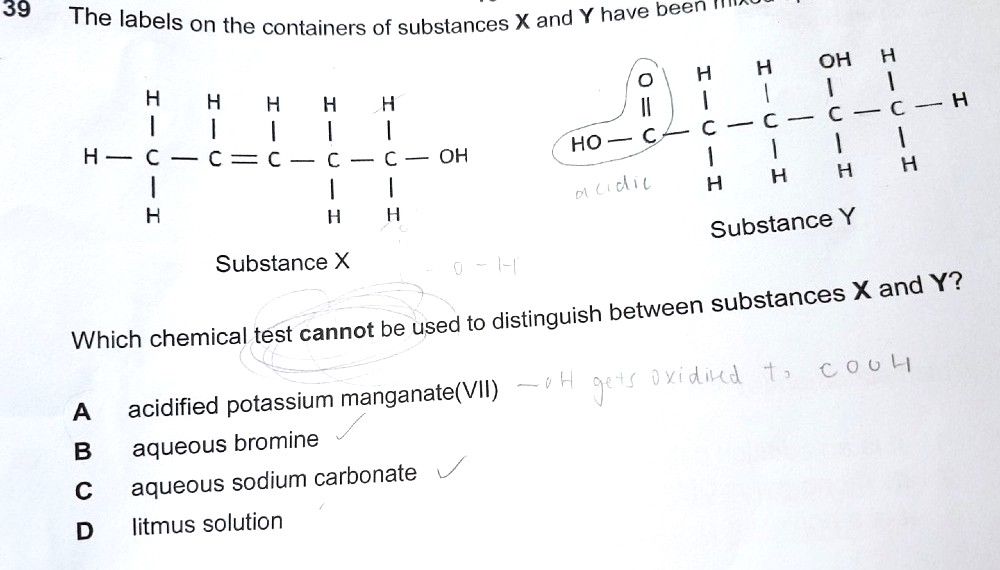
Bromine decolourises the C = C double bond in X, but does not decolourise Y (it does not react with the C = O double bond).
Sodium carbonate reacts with Y and gives off effervescence, even though Y is a relatively weak acid, but it has no visible reaction with X.
For the same acid reason, Y turns litmus red, but it will not show a red result with X.
(In actual fact, I think X is also slightly acid due to the alcohol group itself, but this is not taught at your O levels).
For both, there is no group to stabilise the conjugate base upon dissociation (unlike phenol, where the phenolate ion is resonance stabilised), so the conjugate base is very unstable due to the negative charge on the O- being unable to be dispersed. So dissociation is highly disfavoured and both's alcohol group would be considered neutral.
The answer is A as both X and Y 's alcohol groups will be oxidised to ketone for Y and carboxylic acid for X. Decolorisation of purple acidified KMnO4 occurs so we can't distinguish between the two.
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/13%3A_Structure_and_Synthesis_of_Alcohols/13.05%3A_Acidity_of_Alcohols_and_Phenols
For further reading
Otherwise, he would have wrongly concluded that option A could be used to distinguished between the two compounds
If he only has the knowledge that primary OH can be oxidised to COOH , he would conclude that Y cannot be oxidised as it doesn't have primary OH (not having the knowledge secondary OH is able to be oxidised as well). Then he would conclude there's no decolorisation here.
But X has a primary alcohol so he would think yes, it can be oxidised to COOH and result in decolorisation.
He would then think that A is not the option to choose since he would conclude A can be used to distinguish between the two.
Then since the other 3 options work as well, he would think that none of them are the answers to pick and end up being stuck.
I'm just thinking that from his other comment, it seems to imply that he doesn't know about oxidation of secondary OH to ketones (resulting in decolorisation). If he is from a IP or SAP school that teaches this, then he wouldn't have a problem answering it, unless his content mastery isn't there yet.
I'm guessing the student is on the O level track, but the question is a higher order question that expects the student to make an educated guess about the oxidation of secondary OH.
They're not taught about ketones yet, but would know that oxidation of primary OH 'removes' 1 H, 'turns' the other H into OH and the C-O bond becomes C = O, and thus can use this info to deduce that Y's OH is oxidisable as well, just that it doesn't have the extra H to be 'turned' into OH
See 1 Answer
When you proceed to the A Levels, you will learn that substances such as X is called a primary alcohol, while Y is called a secondary alcohol. This is because the carbon attached to an OH group in X is attached to two other H as well, while the carbon attaches to an OH group in Y is attached to one other carbon (non-H) and one H.
Potassium manganate will be able to react with both primary alcohols and secondary alcohols, but not with what we call tertiary alcohols, where the carbon attached to the OH group is not directly attached to any H.
Primary alcohols get oxidised to carboxylic acids, while Secondary alcohols gets oxidised to something called ketones. Ketones are not covered in your syllabus.
Also, it should be purple with X , red with Y instead of the other way round which it was written
It does this first via dehydration of the alcohol to the alkene, then subsequent oxidation to the carboxylic acid results. You will learn this at university level chemistry.