Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | A Maths
No Answers Yet
Help katherine! Anyone can contribute an answer, even non-tutors.

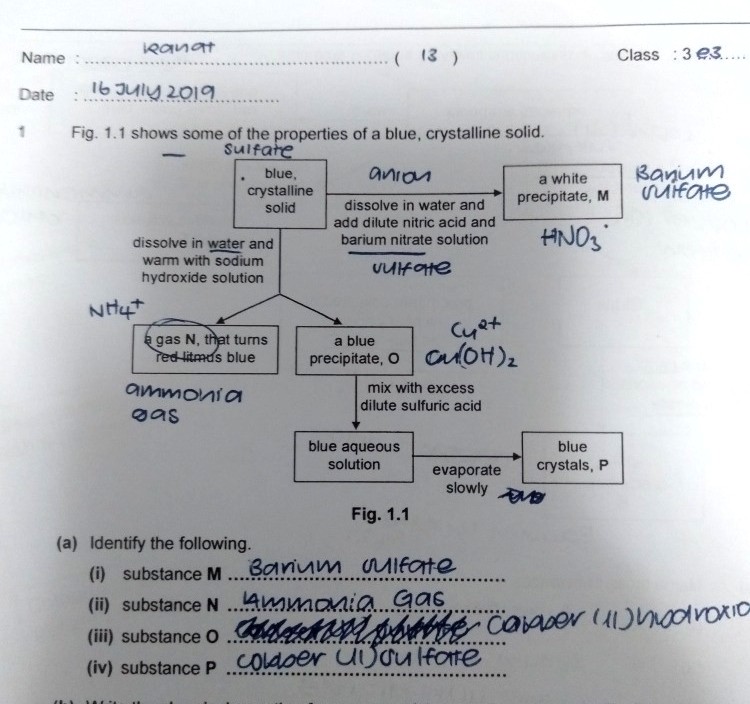
chemistry help !! thank u
I failed to observe the "mix with excess dilute sulphuric acid" part after O.
Okay, here's the breakdown.
1. "Dissolve in water, add nitric acid and barium nitrate" is a test to see whether sulphate is present in the solid or not. The white precipitate formed, M, must be barium sulphate, BaSO4. The blue crystalline solid must contain a sulphate anion.
2. On the other hand, the same blue crystalline solid is allowed to dissolve in water and warmed with NaOH. A gas is formed which turns red litmus paper blue. The only gas capable of turning red litmus blue is ammonia. N must be ammonia gas, NH3. The blue crystalline solid must contain ammonium cation, NH4+.
3. The blue colour of the crystalline solid must have come from copper (II), since copper (II) is the only substance which produces such blue colours. Additionally, this solid must contain ammonium ions as part of a mixture in the solid (though this is not important for this question). Not forgetting the sulphate ions which we have found earlier.
4. The blue precipitate O is formed because the solid reacts with the sodium hydroxide as well. The blue precipitate O must contain the copper (II) ions and hydroxide ions, since copper (II) hydroxide is insoluble in water. O is copper (II) hydroxide, Cu(OH)2.
5. This copper (II) hydroxide is reacted with excess sulphuric acid to produce copper (II) sulphate solution (copper (II) sulphate is soluble in water), which is evaporated to remove significant water content in the solution to leave behind the blue crystals P, copper (II) sulphate.