Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
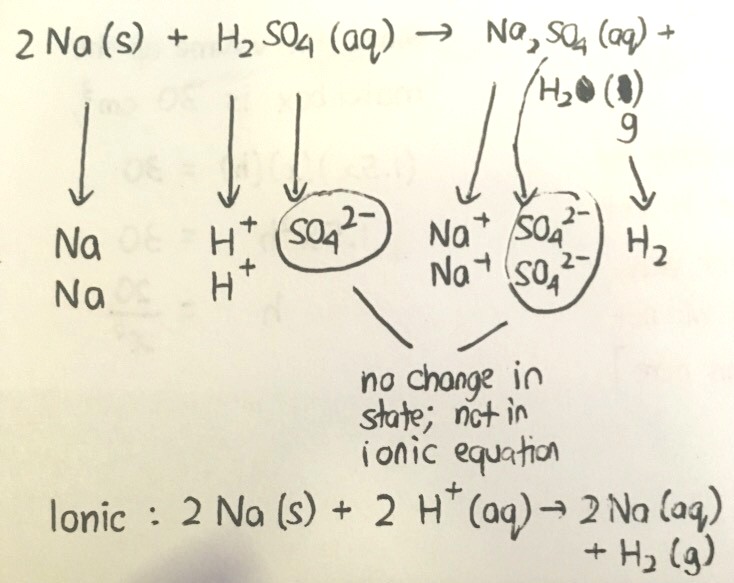
It is very important to be able to list out the state symbols of the respective substances because only substances which change state during a reaction takes part in the ionic equation.
Date Posted:
5 years ago
For your ionic equation, it should be 2Na+ (aq) on the right side instead of 2Na(aq). The charges must be balanced on both sides.
Also, there are species which don't change in state but are included in the ionic equation.
For example, in the halogen displacement reaction,
Cl2(aq) + 2 K+ (aq) + 2 I-(aq) → 2 K+(aq) + 2 Cl-(aq) + I2 (aq)
The ionic equation is :
Cl2 (aq) + 2 I-(aq) → 2 Cl-(aq) + I2 (aq)
Notice the chlorine in Cl2 and 2Cl- are both aqueous but are in the equation. The Cl2(aq) isn't written as split into ions as doesn't dissociate easily due to the nature of the Cl-Cl bond. Only when the displacement occurs does it dissociate to yield the Cl- ions.
The way to spot spectator ions is that if you see the exact same species on both sides, you can cancel them out.
Further info :
some catalysts are known to have changes in physical state both during at and the end of the reaction, though they are chemically unchanged (actually restored to their original chemical form) at the end. And catalysts aren't neither included in the reaction equation nor the ionic equation
Also, there are species which don't change in state but are included in the ionic equation.
For example, in the halogen displacement reaction,
Cl2(aq) + 2 K+ (aq) + 2 I-(aq) → 2 K+(aq) + 2 Cl-(aq) + I2 (aq)
The ionic equation is :
Cl2 (aq) + 2 I-(aq) → 2 Cl-(aq) + I2 (aq)
Notice the chlorine in Cl2 and 2Cl- are both aqueous but are in the equation. The Cl2(aq) isn't written as split into ions as doesn't dissociate easily due to the nature of the Cl-Cl bond. Only when the displacement occurs does it dissociate to yield the Cl- ions.
The way to spot spectator ions is that if you see the exact same species on both sides, you can cancel them out.
Further info :
some catalysts are known to have changes in physical state both during at and the end of the reaction, though they are chemically unchanged (actually restored to their original chemical form) at the end. And catalysts aren't neither included in the reaction equation nor the ionic equation
Oops