Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

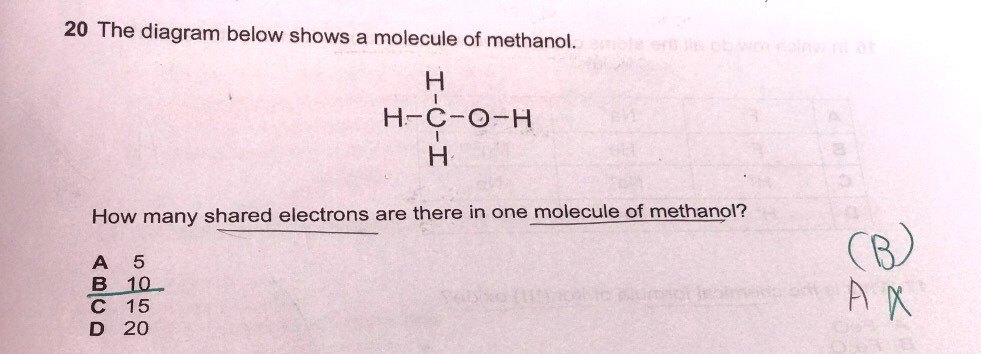
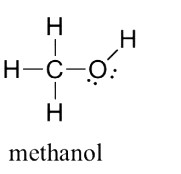
Can someone please explain to me why for this question the answer is 10 I really do not understand what is shared electrons and what do they mean by how many shared electrons are there in one molecule what is one molecule? please someone explain to me this qn thank you very much appreciated
In covalent bonding, electrons are shared amongst atom. In typical situations, equal number of electrons comes from each of the atoms.
See 1 Answer