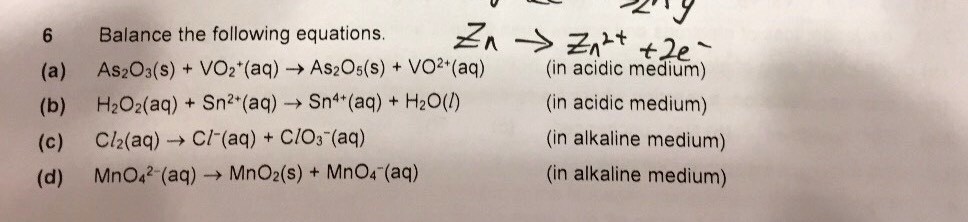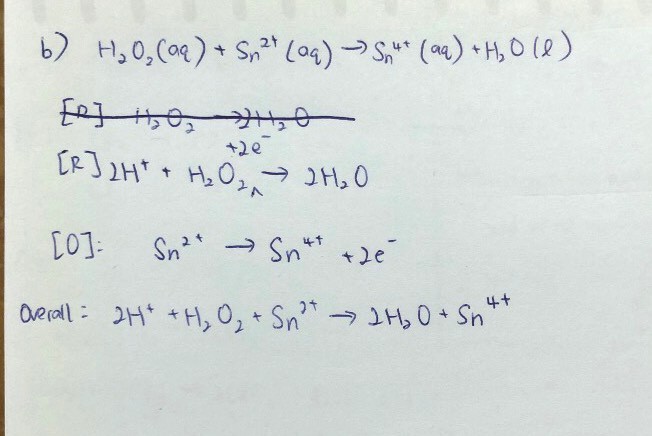Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 4 Answers
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

First step: identify species that is oxidised and reduced;
Second step: split into oxidation and reduction half equations;
Third step: perform AOHE (elaboration in pic) ;
Fourth step: combine the 2 half equations and simplify
Second step: split into oxidation and reduction half equations;
Third step: perform AOHE (elaboration in pic) ;
Fourth step: combine the 2 half equations and simplify
Date Posted:
5 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

Reduction half equation was condensed into 1 line for this qn. It can also be found in the data booklet.
Date Posted:
5 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

In alkaline medium, after adding H⁺ to balance hydrogen, add OH⁻ to both sides
Date Posted:
5 years ago
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes

Reduction half equation was condensed to one line in blue. Full explanation is in red. For species that undergo disproportionation, like in c and d, (same species get reduced and oxidised), the reduction and oxidation half equations start with the same species.
Date Posted:
5 years ago