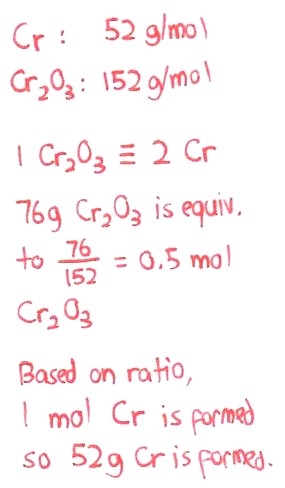Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Q4 my answer is B based on my working.
Q5 my answer is C. Electrolysis works for ionic compounds. Crystallisation, distillation and filtration are physical techniques, so these cannot be used for the chemical splitting of ionic compounds into their respective elements.
Q6 my answer is B. Metal X must be between H and Fe in the reactivity series. Reaction with coke (carbon) is sufficient to extract the metal. There is no need to go all the way to electrolysis to achieve the same result. Heating the oxide will not get the metal out obviously.
So yes, your three answers are correct (or at least, the answers agree).
Q5 my answer is C. Electrolysis works for ionic compounds. Crystallisation, distillation and filtration are physical techniques, so these cannot be used for the chemical splitting of ionic compounds into their respective elements.
Q6 my answer is B. Metal X must be between H and Fe in the reactivity series. Reaction with coke (carbon) is sufficient to extract the metal. There is no need to go all the way to electrolysis to achieve the same result. Heating the oxide will not get the metal out obviously.
So yes, your three answers are correct (or at least, the answers agree).
Date Posted:
5 years ago