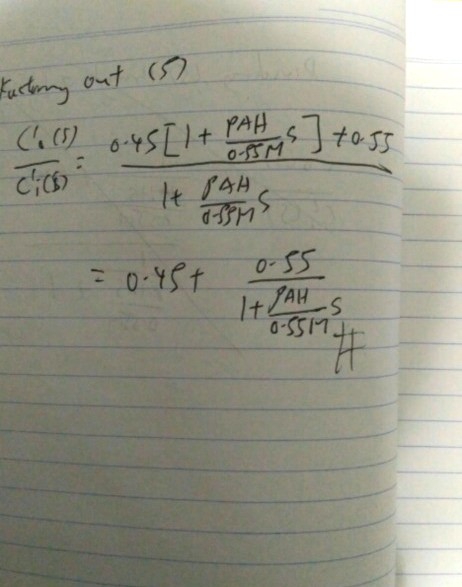Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 2 | H2 Maths
4 Answers Below
Anyone can contribute an answer, even non-tutors.

for Q1 i also have to discuss why higher or lower reflux will give a purer distillate
for Q2 i also have to suggest the temperature on each tray is different
See 4 Answers